Molecules containing urea and thiourea groups are well known in supramolecular chemistry to self assemble into chains via hydrogen bonding interactions, which can be broken by interaction with ions or polar molecules.
Pritam Mukhopadhyay’s group in New Delhi have found that, with the right functionalization, this can lead to interesting optical properties in solution.
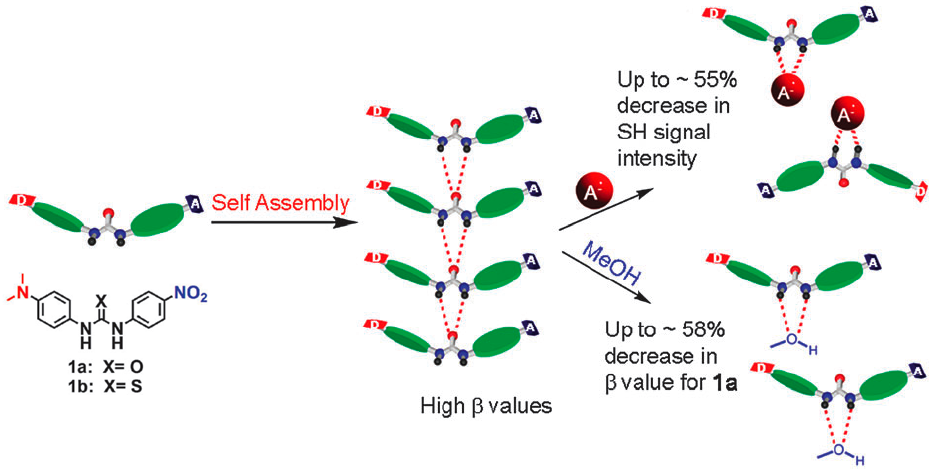
Molecules such as 1a and 1b were found to have non-linear optical (NLO) behaviour in THF solution. On adding a polar molecule such as methanol, or a strongly coordinating anion such as acetate, the NLO behaviour was reduced. This corresponds to the self-assembled urea chains being disrupted by adding a guest that can compete for hydrogen bonding to the urea NH groups.
With this work, the authors have identified an effective new method to probe the assembly and disassembly of supramolecular architectures.
For more information, you can download the full article (free for a limited time) here.










