Commodity chemicals are often produced using catalysts. Despite the many advantages of using catalysts (such as faster conversion, improved selectivity) a major difficulty is separating them from the product at the end of the reaction. Such is the significance of this problem, heterogeneous catalysts are often chosen ahead of their homogeneous brothers because they are simpler to remove at the end of the reaction, despite the homogeneous catalysts generally having better performance.
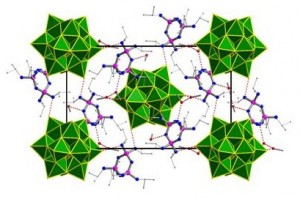
An alternative solution to the separation problem is to utilise a biphasic solvent system. Partitioning the catalyst and product into different phases provides inherent separation and removes the need for expensive procedures like distillations. Ivan Kozhevnikov and Alexander Steiner at the University of Liverpool have collaborated to join their respective areas of expertise together and create catalytically active polyoxometalate (POM)-phosphazene aggregates (Figure 1) which can operate in a biphasic environment. Their communication reports rapid oxidation of test substrates by enhancing the transfer of the catalytically active POM across the two phases. Furthermore, the chemistry is “green” as it utilises relatively environmentally benign conditions.
The components of the aggregates are independently soluble in the different phases; therefore defining how this catalyst operates will be paramount to understanding and developing the system further. For example, reporting the performance of the POM or the phosphazene independently in the biphasic system would provide essential support to the claim that these aesthetically pleasing aggregates are responsible for the observed catalytic activity and remove some of the alternative potential sources.
Read the ‘HOT’ Chem Comm article today (Free to access until the 27th of December):
Michael Craven, Rana Yahya, Elena Kozhevnikova, Ramamoorthy Boomishankar, Craig M. Robertson, Alexander Steiner and Ivan Kozhevnikov
Chem. Commun., 2012, 48, Advance Article
DOI: 10.1039/c2cc36793a