Observing what happens when two substances are mixed together is one of the most important foundations of chemistry. However, to take these observations, understand what they mean and then use that knowledge to manipulate and deliberately influence the outcome of a reaction is where the skill and ingenuity of a chemist truly comes to the fore.
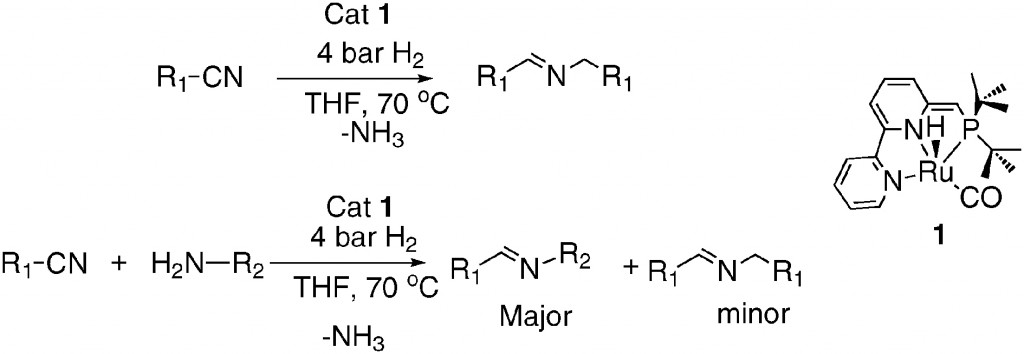
It is with this in mind we can appreciate the work of David Milstein and his co-workers at The Weizmann Institute of Science in Israel. They have shown that nitriles and amines can be coupled using their utilising their versatile “PNN Ru(II) pincer complexes” to produce imines under mild conditions (Scheme 1).
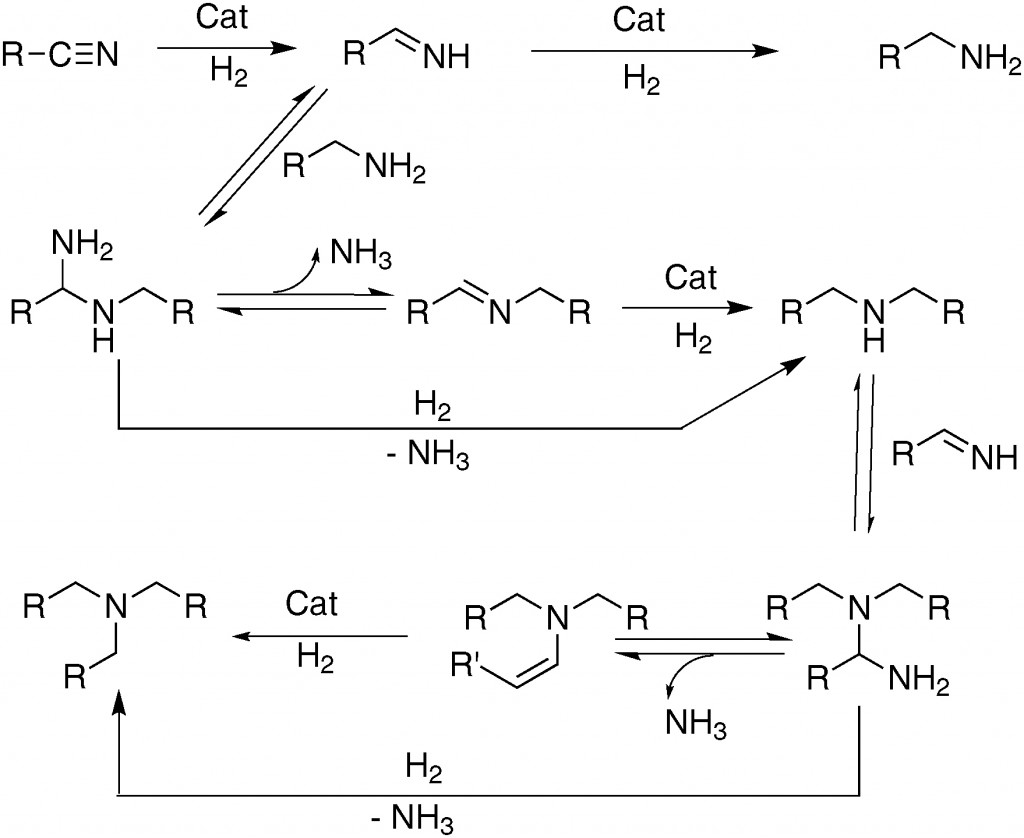
They can control where the reaction stops, which is remarkable as these types of reactions generally yield a mixture of products. We can see why by looking at the mechanism of the reaction (Scheme 2).
Scheme 2 shows why the imine would generally be considered as an intermediate; an unstable compound which readily reacts further, yet in this case it is the product. Isolation of intermediates is incredibly challenging because it involves isolating compounds which are, by their very nature, transient.
The paper shows the reaction works well with hydrogen pressures as low as four bar, perhaps the next step might be to examine just how low the pressure can be decreased. This could potentially remove the necessity for specialized pressurized reaction vessels and may make it the method choice for imine synthesis in almost any lab.
Read the ‘HOT’ Chem Comm article today (Free to access until the 17th of December):
Catalytic coupling of nitriles with amines to selectively form imines under mild hydrogen pressure
Dipankar Srimani, Moran Feller, Yehoshoa Ben-David and David Milstein
Chem. Commun., 2012, 48, 11853-11855
DOI: 10.1039/C2CC36639H