Methods of allylic etherification are usually involved synthetic procedures, due to the need for activation of the starter aliphatic alcohol. By contrast, no such problems exist in analogous reactions to form allylic amines.
Significant steps have been made in this synthetic area, but limitations remain. Iridium catalysis has been successfully employed for this reaction but usually requires the use of a glove box. Derivatisation of starting materials to incorporate suitable leaving groups has also been explored, as has the use of various bases to deprotonate the alcohol nucleophile.
Tertiary butyl alcohol has been extensively investigated for its reactivity also, but it appears that the more conditions you put on this reaction the more limited its scope and applicability become. Add to this the effect of each modification on product regio- and stereoselectivity and you can appreciate the challenge. Each new ‘improvement’ can leave you, figuratively speaking, one step up, and two steps back.
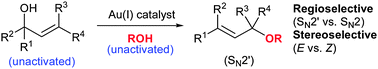
With this situation in mind, the Lee group from Heriot-Watt University report a considerable breakthrough in the catalysis of the allylic etherification of unactivated alcohols, using a gold(I) salt. Well chosen controls and an extensive optimisation of the reaction parameters has yielded a robust strategy, effective for a wide range of substrates and aliphatic alcohols, with very good to excellent regio- (SN2 vs SN2′) and stereoselectivity (cis/trans). An inert atmosphere is not even required.
The use of Lewis acidic gold(I) for this reaction seems to provide a reliable activation of the olefin of the allyl group, to nucleophilic attack from the external alcohol nucleophile, all helped along by the possible involvement of a six-membered transition state. It would appear that it is this activation and plausibly reliable mechanism, that has allowed this usually difficult process to be controlled, both chemically and stereochemically.
A large variety of alcohol nucleophiles, (including primary, secondary, tertiary, and functionalised examples) and allyl alcohol electrophiles have been screened. Overall trans- products with SN2′ regiochemistry are highly favoured. An interesting selection of control reactions were performed, including the use of a hindered proton sponge to prove absolutely the effect of the gold(I) catalyst as well as the sole use of the acidic bis-trifluoromethylsulfonimide (HNTf2) as catalyst, which resulted in a conversion of less than 5%.
The authors report a breakthrough in the allylic etherification of aliphatic alcohols, the application of which should be substantial, as the team now turns its attention to possible applications in asymmetric synthesis.
Read the ‘HOT’ Chem Comm article today (Free to access until the 14th of December):
Gold(I)-catalysed direct allylic etherification of unactivated alcohols
Paul C. Young , Nina A. Schopf and Ai-Lan Lee
Chem. Commun., 2012, 48, Advance Article
DOI: 10.1039/C2CC36760B
Published on behalf of Kevin Murnaghan, Chemical Communications web science writer.