Researchers from the University of Geneva have developed a transition-metal-free method for the asymmetric installation of fluoroalkyl groups.
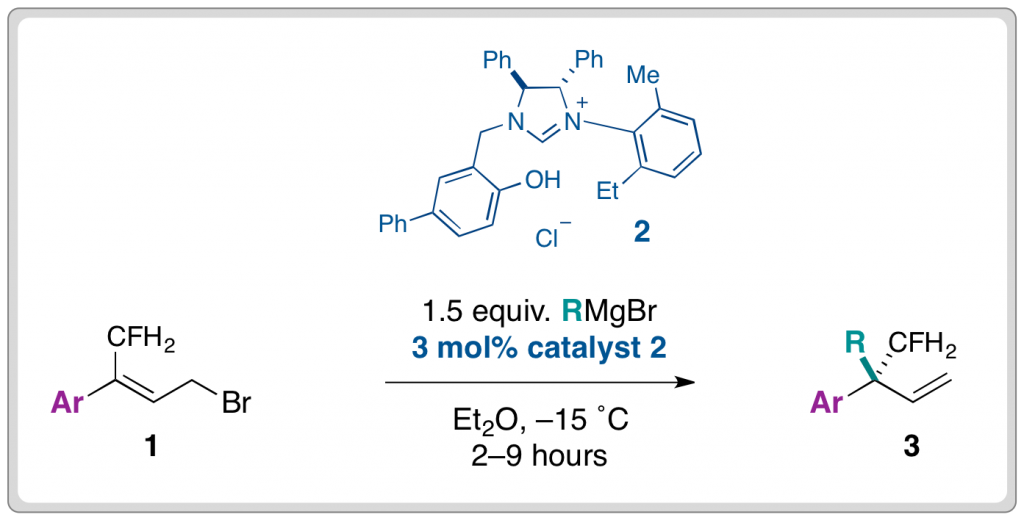
The Alexakis group found that the use of N-heterocyclic carbenes (2) in conjunction with Grignard reagents, enabled the highly selective synthesis of γ-functionalised products (3) from readily accessible starting materials (1). The reaction was initially performed in the presence of copper(l) salts, which afforded the desired products although regioselectivity and ee were suppressed.
A range of alkyl groups (R) could be introduced in moderate yields and very good levels of enantioselectivity (84–95%) from the corresponding Grignard reagent. Similarly, the reaction was tolerant of different aryl substituents (Ar). Exchanging the aryl component with cyclic alkyl groups did not affect the ee, however, the introduction of less bulky aliphatic groups caused enantioselectivity to plummet.
The demonstrated importance of fluoroalkyl groups in medicinal chemistry necessitates the development of new methods for the introduction of this important functional group. Alexander Alexakis and his group have developed the first asymmetric allylic alkylation reaction for the synthesis of quaternary centres containing fluoroalkyl groups; a method that may be of particular benefit to the pharmaceutical industry.
Read this HOT Chem Comm article today (free to access until the 13th of December 2012):
Formation of chiral fluoroalkyl products through copper-free enantioselective allylic alkylation catalyzed by an NHC ligand
David Grassi , Hailing Li and Alexandre Alexakis
Chem. Commun., 2012, 48, 11404-11406










