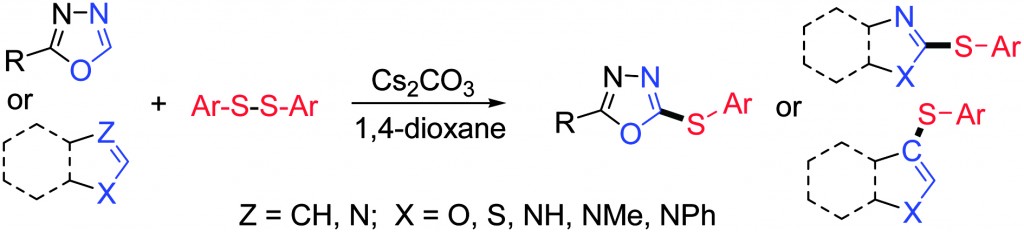
Aromatic compounds substituted with a C-S bond are of importance for pharmaceutical and medicinal chemistry, and materials science. A widely employed route to such compounds is a cross coupling reaction, between an aryl halide and a thiol, to yield a thioether. The reaction is usually mediated and catalysed by a metal centre such as palladium, indium or nickel, present as a salt or complex. In this communication, the authors report a high yielding, transition metal free route to aryl thioethers, using just a disulfide and a weak base, in a suitable solvent, under an inert gas.
The authors proved early in their study that a metal salt was not required for reactivity, with better yields being recorded for such reactions, versus a metal salt containing control. A further control reaction was carried out, to disprove the presence or effect of any trace transition metal by using ultrapure caesium carbonate (99.994% purity) and freshly distilled solvent.
The model reaction investigated and optimised was the thiolation of 2-phenyl-1,3,4-oxadiazole with di-p-tolyl disulfide. Reaction conditions which gave the best yields involved the use of 2 equivalents of base and 1,4-dioxane as solvent, under an argon atmosphere, for typically 18-24 h. 5 equivalents of disulfide were found to be the most effective. Using 7 equivalents gave no benefit to the final yield. Coincidently, the excess disulfide proved easily recoverable from the reaction mixture.
Electronic effects proved to be important in the reactivity of the phenyl-oxadiazoles with electron donating groups present on the arene promoting the reaction and electron withdrawing groups having a negative effect. The reaction also shows a good degree of robustness in being effective in thiolating indole and 5-methyl-indole at the 3 positions in excellent yield, as well as other substrates such as caffeine and benzimidazole.
This synthetic methodology represents an important simplification in the preparation of heteroaromatic thioethers, and should prove of interest to synthetic chemists, particularly in the areas of medicinal chemistry, materials science and total synthesis.
Read this HOT Chem Comm article today (free to access until the 7th of December 2012):
Transition metal-free direct C–H bond thiolation of 1,3,4-oxadiazoles and related heteroarenes
Liang-Hua Zou, Jens Reball, Jakob Mottweiler and Carsten Bolm, Chem. Commun., 2012, 48, 11307–11309
Published on behalf of Kevin Murnaghan, Chemical Communications web science writer.