Synthetic organic chemists have long regarded the Wittig reaction as one of the most significant methods for the creation of new C–C bonds. Now, researchers at Peking University have reported that this time-honoured reaction can be used for the modification of proteins in a bioorthogonal process.
Bioorthogonal processes represent a growing area of interest, encompassing reactions which can take place under physiological conditions. That is, the reaction must proceed at neutral pH in an aqueous solvent at ambient temperature and low concentrations, with high selectivity. Given these challenging parameters, the range of bioorthogonal processes remains limited.
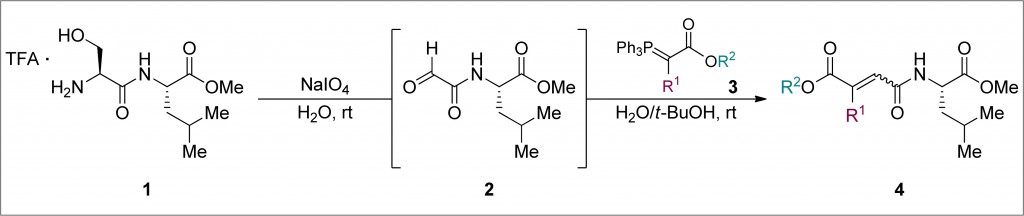
The Ye group, based at the Key State Laboratory of Natural and Biomimetic Drugs, have contributed to the expansion of this fascinating area of chemistry by successfully applying the Wittig reaction to their one-pot site-selective protein modification. The first step involves the incorporation of an aldehyde at the N-terminus of a peptide chain (1). This could be achieved by the periodate oxidation of N-terminal serine or threonine residues, or by PLP (pyridoxal-5-phosphate) oxidation of N-terminal glycines. The resulting aldehyde (2), without the need for isolation or purification, could be reacted with an ylide (3) to form a wide variety of functionalised peptide products (4). Di-, penta- and hexa-peptide substrates could be functionalised in this manner, using water and t-butanol as co-solvents at room temperature.
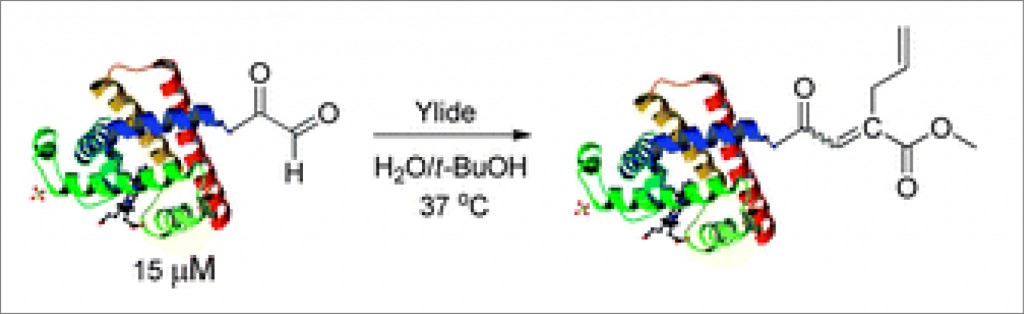
The strength of this site-selective reaction was further demonstrated by modifying myoglobin. Crucially, this was achieved with no damage to the protein’s secondary or tertiary structure and, furthermore, Prof. Ye’s group established that myoglobin’s oxygen storage and release function was unaffected.
The functionality introduced offers the potential for further structural modification, or for use in medical imaging. With protein-based pharmaceuticals becoming widely used, greater insight into protein function and behaviour is of paramount importance. This methodology has the potential to be a valuable tool in that understanding.
Read the ‘HOT’ Chem Comm article today:
Enabling Wittig reaction on site-specific protein modification
Ming-Jie Han, De-Cai Xiong and Xin-Shan Ye
Chem. Commun., 2012, 48, 11079-11081
DOI: 10.1039/C2CC35738K
Published on behalf of Ruth Gilligan, Chemical Communications web science writer.