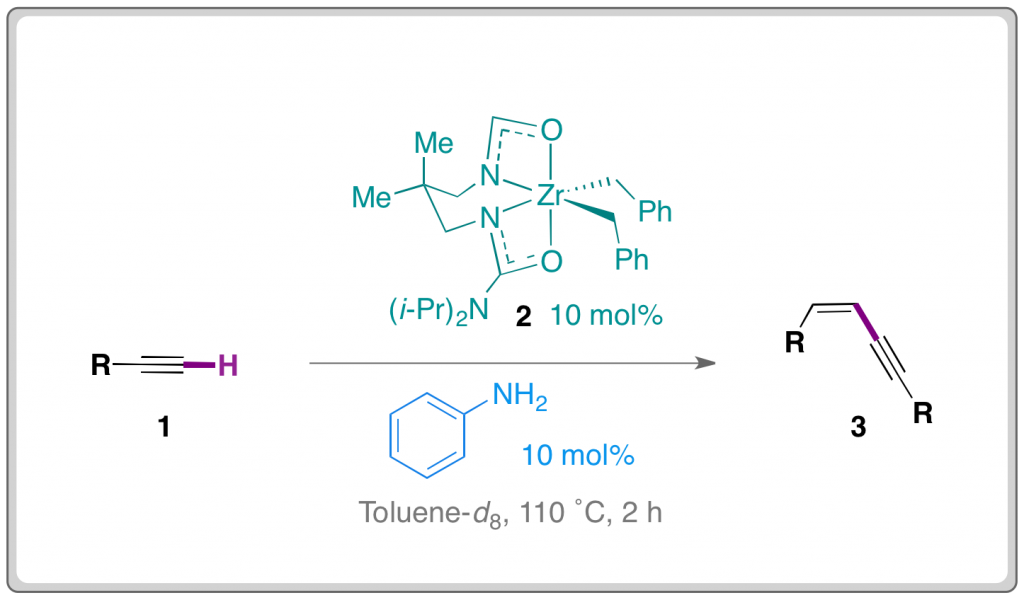
Researchers from the University of British Columbia have developed a ‘head to head’ dimerisation of alkynes to provide (Z)-enynes in high yields.
The conjugated enyne products are important, both as intermediates for organic synthesis and in optoelectronics – a type of electronics that detect, source and control light. Therefore, new methods for the selective synthesis of these compounds are in demand.
Platel and Schafer have overcome regioselectivity problems often associated with alkene dimerisation. The researchers used a readily accessible Zirconium catalyst (2) in conjunction with aniline as co-catalytic proton source.
 A number of alkynes (1) were successfully dimerised to provide (Z)-enynes (3) in mostly high yields. The reaction is tolerant of a number of differentially substituted aryl compounds and also some alkyl rings.
A number of alkynes (1) were successfully dimerised to provide (Z)-enynes (3) in mostly high yields. The reaction is tolerant of a number of differentially substituted aryl compounds and also some alkyl rings.
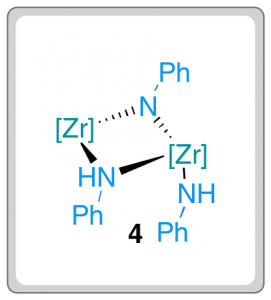
The exclusive formation of (Z)-enynes was at odds with previously established mechanistic pathways for metal-catalysed alkyne dimerisation and led the researchers to further probe the mechanism. Complex 4 was isolated when a stoichiometric amount of aniline and complex 2 were mixed, leading the researchers to consider the validity of 4 as a catalytic intermediate. When 4 was mixed with alkyne (1) under the reaction conditions, complete conversion to (Z)-enyne (3) was observed. This result suggests that the unusual dimeric imido-bridged species is a viable intermediate in the reaction pathway.
This method represents an interesting development in catalytic and regioselective C–C bond formation.
Read the ‘HOT’ Chem Comm article today:
Zirconium catalyzed alkyne dimerization for selective Z-enyne synthesis
Rachel H. Platel and Laurel L. Schafer
Chem. Commun., 2012, 48, 10609-10611
DOI: 10.1039/C2CC35913H