Non-covalent interactions dictate the assembly of many of nature’s most elegant structures. Similarly, supramolecular chemists have long been intrigued by the challenge of designing functional structures that spontaneously self-assemble from simpler fragments which mutually recognise each other.
A popular self-assembly approach is to produce coordination compounds from transition metal salts with rigid organic ligands. Directional bonding around transition metal centres allows the production of predictable and controllable shapes. Michael Schmittel’s group at the University of Siegen have been exploring a newer approach. They prepared two assemblies, a 2-component triangle T1 and a 3-component rectangle R1. The transition metal “corner” arrangements in T1 and R1 are disfavoured, so if the two assemblies are combined the components re-shuffle to form a more favourable assembly- the 5-component triangle T2. The transformation occurs at room temperature, and can be completed in just 1 hour in the presence of a catalyst, which accelerates the re-shuffling by labilising the metal-ligand bonds.
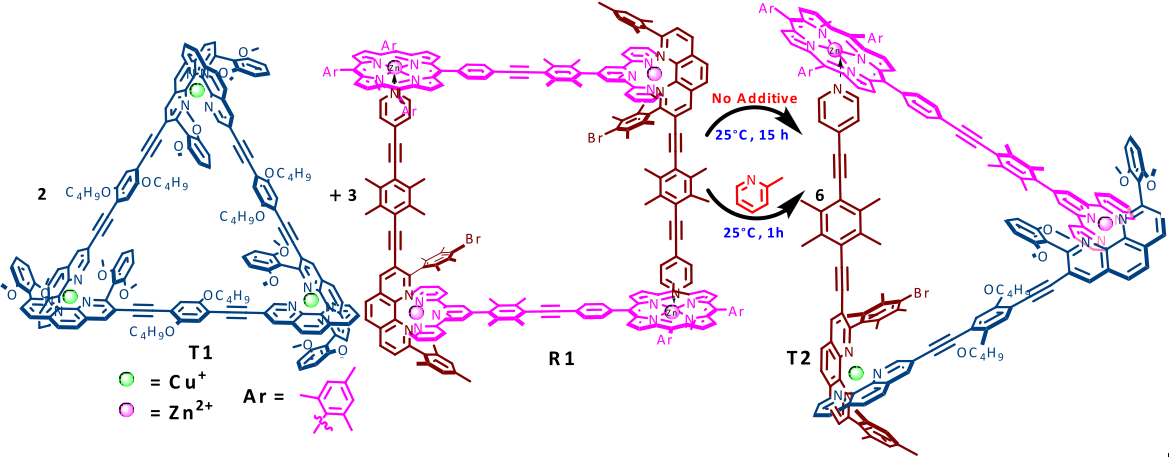
Unlike previous examples, the conditions needed for the transformation are very mild. The authors compare the process to gene shuffling, the combination of dissimilar genes to form new genetic material. The strategy could be considered a first step towards the evolution of supramolecular architectures, and a great route to more complex supramolecular assemblies with higher information content.
The full communication can be downloaded here (free to access for a limited period).
Cally Haynes










