This month sees the following articles in ChemComm that are in the top ten most accessed:-
Control the size and surface chemistry of graphene for the rising fluorescent materials
Shoujun Zhu, Shijia Tang, Junhu Zhang and Bai Yang
Chem. Commun., 2012,48, 4527-4539, DOI: 10.1039/C2CC31201H
A selective reaction-based fluorescent probe for detecting cobalt in living cells
Ho Yu Au-Yeung, Elizabeth J. New and Christopher J. Chang
Chem. Commun., 2012,48, 5268-5270, DOI: 10.1039/C2CC31681A
Palladium-catalyzed direct phosphonation of azoles with dialkyl phosphites
Chaodong Hou, Yunlai Ren, Rui Lang, Xiaoxue Hu, Chungu Xia and Fuwei Li
Chem. Commun., 2012,48, 5181-5183, DOI: 10.1039/C2CC30429E
Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices
Jianhua Shen, Yihua Zhu, Xiaoling Yang and Chunzhong Li
Chem. Commun., 2012, 48, 3686-3699, DOI: 10.1039/C2CC00110A
A homochiral metal-organic framework membrane for enantioselective separation
Wenjin Wang, Xueliang Dong, Jiangpu Nan, Wanqin Jin, Zhongqiao Hu, Yifei Chen and Jianwen Jiang
Chem. Commun., 2012, Advance Article, DOI: 10.1039/C2CC32595K
Novel catalytic effects of Mn3O4 for all vanadium redox flow batteries
Ki Jae Kim, Min-Sik Park, Jae-Hun Kim, Uk Hwang, Nam Jin Lee, Goojin Jeong and Young-Jun Kim
Chem. Commun., 2012,48, 5455-5457, DOI: 10.1039/C2CC31433A
A charge transfer assisted fluorescent probe for selective detection of hydrogen peroxide among different reactive oxygen species
Manoj Kumar, Naresh Kumar, Vandana Bhalla, Parduman Raj Sharma and Yasrib Qurishi
Chem. Commun., 2012,48, 4719-4721, DOI: 10.1039/C2CC30932G
Recent efforts directed to the development of more sustainable asymmetric organocatalysis
José G. Hernández and Eusebio Juaristi
Chem. Commun., 2012,48, 5396-5409, DOI: 10.1039/C2CC30951C
Palladium-catalyzed monoselective C-H borylation of acetanilides under acidic conditions
Bin Xiao, Yi-Ming Li, Zhao-Jing Liu, Han-Yi Yang and Yao Fu
Chem. Commun., 2012,48, 4854-4856, DOI: 10.1039/C2CC31737K
Transfer of chirality from ligands to metal centers: recent examples
Jeanne Crassous
Chem. Commun., 2012, Accepted Manuscript, DOI: 10.1039/C2CC31542D
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to ChemComm? Then why not submit to us today or alternatively contact us with your suggestions.











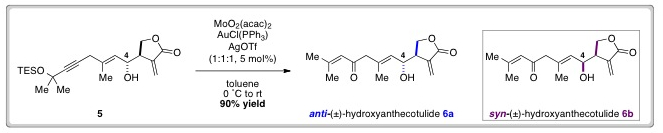

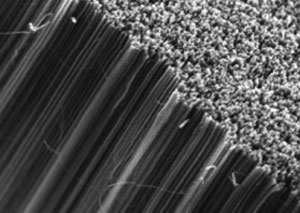 3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?
3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?