Phthalocyanines are important industrial oxidation catalysts that are cheap and easy to make but their mechanism remains unclear. Now a team of French and Russian scientists have made and characterised a key intermediate in the catalytic cycle, previously postulated but never obtained.
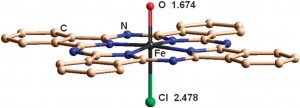
Pavel Afanasiev and Alexander Sorokin from the CNRS-Université Lyon, France, and colleagues prepared and characterised the first high-valent iron oxo species on the phthalocyanine platform.
They treated tetra-tert-butylphthalocyanine iron chloride with m-chloroperbenzoic acid to give what they later proved to be an Fe(IV) oxo species. Isolation of such a reactive species is extremely challenging so the team characterised the reaction mixture in situ shortly after mixing the reagents. This approach also removed the possibility of the molecule undergoing further transformation en route to the X-ray diffraction facility or NMR spectrometer.
The team used at least eight different techniques – including mass spectrometry, UV-vis and EPR spectroscopy, density functional theory (DFT) and X-ray emission studies – in their incredibly thorough examination of the molecule, not only confirm its creation but to fully define many aspects of its electronic structure. These results provide a platform from which a better understanding of iron phthalocyanine catalysts can be developed.
Keen to read more? Download this ChemComm article here or visit our web collection on Porphyrins & Phthalocyanines.
Posted on behalf of Ruaraidh McIntosh, Chemical Communications web writer.