Southampton chemists, lead by Richard Whitby, have completed the first total synthesis of the marine metabolite mucosin. As the synthesis is enantioselective (they made the (+) enantiomer), the team were able to determine the absolute stereochemistry of the natural compound (the (-) enantiomer), which was isolated from a sponge in the Mediterranean in 1997.
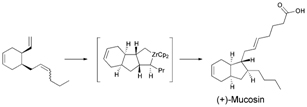 Of particular note in the synthesis is the use of a zirconium-induced co-cyclisation to install the stereochemistry of the four contiguous stereocentres around the unusual bicyclo(4.3.0)nonene core.
Of particular note in the synthesis is the use of a zirconium-induced co-cyclisation to install the stereochemistry of the four contiguous stereocentres around the unusual bicyclo(4.3.0)nonene core.
Read more about the work in their communication, which is free to download for a limited period.
If you’re interested in natural products, check out Natural Product Reports, which just published its regular and popular Marine Natural Products review article.










