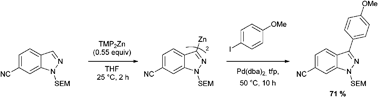
Paul Knochel from the Ludwig Maximilians University in Munich has developed a method to directly metalate and then functionalise N-protected indazoles.
Indazole heterocycles are important molecules in medicinal chemistry and methods to functionalise them are widely sought after. Direct metalation of indazoles at their 3 position is often problematic as this can quite easily lead to ring opening and formation of an aminonitrile product. Knochel overcame this problem by using a zinc reagent to form a bis-indazoylzinc compound.
The bis-indazoylzinc compound reacts with a wide range of electrophiles and can also undergo arylation in a Negishi cross-coupling reaction. In general, such reactions are not possible using normal metalation reagents. Future work will concentrate on the synthesis of biologically active molecules using this methodology.
If you want to find out more then download the ChemComm article, free for a limited period.