When deciding which material to use for a particular application, it’s often necessary to weigh up the pros and cons of each candidate. Wouldn’t it be great if you could combine the best bits from each one to produce the ideal material?
This is exactly what Marco Fraaije and his team from the University of Groningen did to create a new monooxygenase enzyme capable of performing Baeyer–Villiger oxidations with ultimate catalytic properties. For biocatalytic applications, enzymes need to be robust and should ideally be able to catalyse a broad range of substrates. Unfortunately, the only monooxygenase shown to be thermally stable (phenylacetone monooxygenase, PAMO) has narrow substrate specificity. On the other hand, there is cyclohexanone monooxygenase, CHMO, which can oxidise hundreds of substrates yet cannot be used at elevated temperatures.
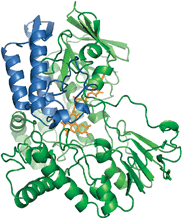
The monooxygenase. Original PAMO structure is shown in green; the replaced sub-domain is shown in blue.
By replacing the substrate-binding domain of PAMO with that of CHMO or steroid monooxygenase (STMO), Fraaije was able to engineer an enzyme that was thermally robust and able to accept a wide range of substrates. Not only were the team able to combine the best of both worlds but in some cases, supersede them as they found when evaluating the conversions and enantiomeric excesses. It seems that the enzyme blend is not necessarily an average of the parent enzymes but can exhibit new properties.
Read the ChemComm article to find out more on how the team were able to improve the properties of Baeyer–Villiger monooxygenases.
Also of interest… ChemComm‘s Enzymes and Proteins web theme issue guest edited by Professors Nicholas Turner, Wilfred van der Donk and Herbert Waldmann.