Researchers from the University of Birmingham have developed a radical cascade process for rapid access to intermediates, which are important for the synthesis of alkaloids related to the stephacidin family.
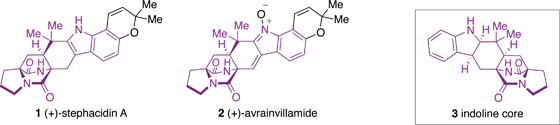
Stephacidin A (1), along with other structurally related compounds, has been shown to possess activity against a number of tumour cell lines.
The group of Nigel Simpkins have demonstrated an elegant approach to the synthesis of the indoline core 3, present in a number of naturally occurring alkaloids.
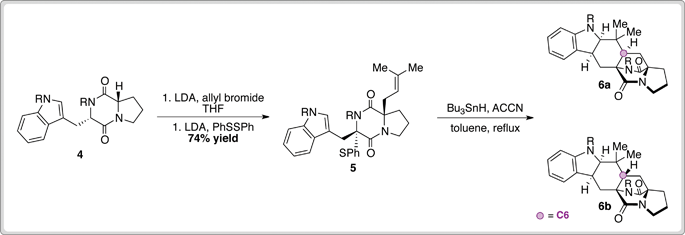
Commencing from 4 (available in five steps from tryptophan), a one-pot prenylation and sulfenylation sequence afforded cyclisation precursor 5 in good yield. Treatment of sulfenyl diketopiperazine 5 with Bu3SnH and ACCN facilitated a double radical cyclisation to provide 6a and 6b. Pleasingly, isomers possessing the correct C6 stereochemistry represented the major products (4.6–3:1 depending on R group), and desired product 1 could be obtained following deprotection of 6a and 6b and subsequent purification.
This work represents an efficient entry into the stephacidin alkaloids and a potentially powerful method for the synthesis of other medicinally relevant analogues.
To read more on Simpkin’s indoline synthesis, download the ChemComm article.