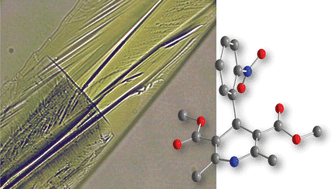
Identification of all solid forms of a pharmaceutical is important for drug delivery, due to the potential variability in the physical and chemical properties between amorphous and crystalline forms. The amorphous form of a compound is typically more soluble, but less stable than its crystalline counterpart. Not only that but different polymorphs of the crystalline compound can also have significantly different properties. To accurately characterise drug action, these polymorphs need to be identified.
Using small-scale crystallisation and in-situ Raman spectroscopic analysis of the antihypertensive drug, nifedipine, Franziska Emmerling and colleagues discovered an extraordinarily fast transition from the glassy amorphous state to the metastable β polymorph in less than a minute. The β polymorph is stable for less than ten minutes before transforming again, to the thermodynamically stable α polymorph.
The speed at which the transformations take place implies that classical diffusion is not responsible for the different polymorphs but could instead be the result of small intramolecular changes arising from a pre-ordered physical arrangement of the molecules.
In an industrial world where screening for solid drug forms is always leaning towards scale-reduction and time-reduction, three physical forms on a glass slide in less than twenty minutes is pretty impressive!
To find out more, download the ChemComm article.
Also of interest… Read Andrew Bond, U. Ramamurty, and Gautam Desiraju’s Chemical Science article on “Interaction anisotropy and shear instability of aspirin polymorphs established by nanoidentation“.
Posted on behalf of Scott McKellar, web science writer for ChemComm.