N-heterocyclic carbenes (NHCs) are extremely useful reagents in synthesis and catalysis, but unfortunately they are expensive with 1 gram costing several hundred US dollars.
However, as Ulrich Siemeling and his colleagues report in their latest ChemComm, it seems that a much cheaper alternative is on the horizon…
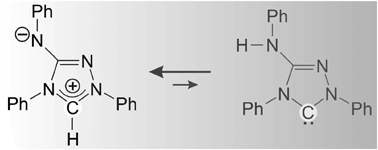
The team of scientists based at the University of Kassel have discovered that Nitron, a low cost analytical reagent, exhibits surprising NHC reactivity, more akin to that of its tautomeric form than its conventional Lewis structure. By reacting Nitron with typical carbene trapping reagents such as elemental sulphur, CS2, and rhodium complexes, they have proven that it is indeed Nitron’s tautomer that it responsible for its NHC-like reactivity in solution, despite being present at concentrations undetectable by NMR spectroscopy.
At a fraction of the price and already commercially available, Nitron may soon become a very popular choice in NHC reactions.
To read more about how Siemeling and co-workers established the cause for Nitron’s unusual reactivity, download the ChemComm article.