Miquel Solà and Jordi Poater working University of Girona in Spain have put forward an extension of Hirsch’s rule to open-shell spherical species.
The famous Hückel rule allows one to estimate whether or not a planar ring molecule would have aromatic properties. When the molecule has 4N + 2 π-electrons then it follows Hückel’s rule. In 2000 Andreas Hirsch found a rule to predict the aromaticity of fullerenes, known as the 2(N +1)2 rule and now Solà has extended this rule to spherical systems with an open-shell.
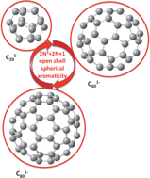
They found that spherical compounds with a half filled last energy level, e.g. those with 2N2 + 2N + 1 electrons, are aromatic. This was backed up with computational evidence and showed that for example C6019+ and C601- both have strong aromatic character.
Solà speculates that this finding could be an important tool for those working in the in stable high spin molecules such as molecular magnets.
Interested in finding out more? Then download the full ChemComm article for free today.










