Peter Roesky, Siegfried Blechert and co-workers at the Karlsruhe Institute of Technology and the Berlin University of Technology have reported the first use of a dizinc complex as the catalyst in a hydroamination reaction.
Hydroamination is the addition of an N–H bond of an amine to an unsaturated C–C bond to give a molecule that contains nitrogen in one step. This is particularly important because many current amine syntheses are multi-step processes. Zinc complexes are advantageous for hydroamination as they are relatively cheap, air and moisture stable and tolerant to a wide variety of functional groups.
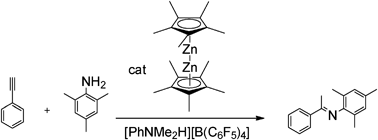
With this in mind Roesky and Blechert decided to test the zincocene complex, Zn2(η5-C5Me5)2, for its hydroamination activity. This complex was discovered in 2004 but it is the first time that it has been used as a catalyst. The team found that the catalyst worked well with good yields and conversions and that it tolerates many functional groups.
Want to find out more? Then download the ChemComm article for free today and leave a comment below.










