Fluoride, in its organic and inorganic forms, has been increasingly present in the food and beverage chain over the years. It has been added to toothpastes to prevent cavities; different forms of fluoride have been included in pesticides; several categories of food, including cereals, have been fortified with fluoride and even water for public consumption has been artificially fluorinated for decades.
Regardless of the debate about its toxicity, it cannot be denied that as such a ubiquitous chemical in the human food chain, the development of simple methodologies and techniques to accurately detect the concentration of this anion in vivo plays an important role in biochemical research.
One of these techniques employs the use of fluorescent indicators that detect the presence of fluoride anions in solution.
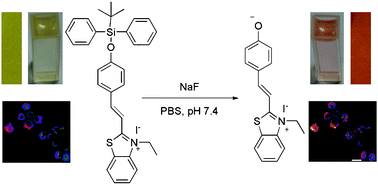
Recent research by the Chinese group of Ma, Du and Zhang has been focused on the realisation of a novel “chemodosimeter” for fluoride that responds to the requisites of ease of synthesis, activity in highly aqueous solutions and buffers, cell permeability, quantitative response and high selectivity.
Their sensor, incorporating benzothiazolium hemicyanine as the fluorophore, was tested in fluorine detection in water:ethanol solutions (7:3) containing phosphate buffered saline at a pH of 7.4. In these conditions, the fluorescence of the sensor was not only quantitatively responsive to changes in fluoride concentration, but showed a change in the fluorescence spectrum, with emission at a different wavelength when in presence of the analyte and even noticeable to the naked eye. The versatility and selectivity of the system was also assessed by performing competition experiments in the presence of other anions, such as CO32-, SO42-, NO3–, Cl–, I– and selected aminoacids and proteins like cysteine and human serum albumin, demonstrating a remarkable preferential response for fluoride.
Tests were also performed on living cells in order to determine the cytotoxicity of the chemodosimeter, using HeLa cells as the test substrate, showing low toxicity under the operational conditions.
The selectivity over different anions and analytes of biological relevance, the ability to operate in strongly aqueous solutions, the reliability and quantitative response and the applicability to living cells may make this new chemodosimeter a beneficial tool for biomedical researchers.
To find out more, read the full article:
A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ions in living cells
Baocun Zhu, Fang Yuan, Rongxia Li, Yamin Li, Qin Wei, Zhenmin Ma, Bin Du and Xiaoling Zhang
Chem. Commun., 2011, DOI: 10.1039/C1CC11308A
Posted on behalf of Dr. Giorgio De Faveri, Web Writer for Catalysis Science & Technology.










