The presence of abnormally high or low level of zinc in specific organs and areas has been linked to several diseases and conditions, including Alzheimer’s disease, stroke and epilepsy. In the light of these considerations, it is not surprising that many efforts have been devoted to in vivo zinc sensing and quantification.
A significant portion of the research to uncover the correlations between the concentration of metal ions, pathologies and development is performed on animal models such as zebrafish (Danio rerio). Due to their fast development, they can be grown outside the mother’s body, and their embryos are transparent, allowing for a clear observation of their organs without the need for dissection.
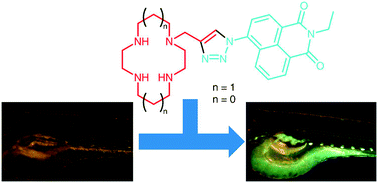
Watkinson and Goldup, from Queen Mary University of London, recently focused their effort to the development of a fluorescent sensor for zinc to be used in model studies on zebrafish. The sensor is based on macrocyclic nitrogen-containing rings (cyclen or cyclam) equipped with a fluorescent pendant arm, introduced using a widely known and applied “click” cycloaddition. The sensor’s fluorescence is activated upon coordination of the zinc anion in a scorpionate fashion.
The sensors proved reliable and stable in a wide range of pH, ideal for in vivo use, and a remarkable selectivity for zinc over other possible anions. Competition experiments with twelve different anions showed that only in the presence of Fe3+ and Cu2+ in threefold excess (in relation to zinc) the sensor failed to discriminate between the metals.
To test their performance in biological models, zebrafish eggs were grown in solutions of the sensors and the distribution of fluorescence monitored during their growth, proving not toxic to the subjects. The accumulation of fluorescence concentrated in the eye, the gall bladder and the biliary system, all regions not highlighted by previous sensors, suggesting a different mechanism of absorption for these macrocyclic compounds and different cell permeability.
Although those reported are preliminary results, the characteristics of these sensors may make them a viable candidate for future applications in vivo sensing of zinc pools.
Read the article online or access the ESI (free).
Modular ‘click’ sensors for zinc and their application in vivo
Kajally Jobe, Caroline H. Brennan, Majid Motevalli, Stephen M. Goldup and Michael Watkinson
Chem. Commun., 2011, 47, 6036-6038
Posted on behalf of Dr. Giorgio De Faveri, Web Writer for Catalysis Science & Technology
This communication is part of the ChemComm Supramolecular Chemistry web themed issue. Check out the web theme page to download other contributions from this exciting issue.










