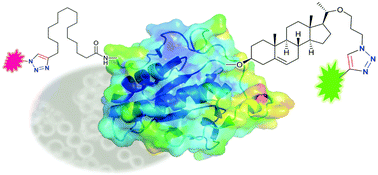
UK chemists have for the first time used a chemical probe to study the post- translational cholesterylation of proteins in living cells.
Post-translational modification (PTM) in cells plays an important role in the function of proteins in vivo. One example is the mammalian Hedgehog (Hh) protein family: the post-translational cholesterylation of Sonic hedgehog (shh) protein regulates its secretion. However, mis-regulation of this protein can promote different types of cancers. Therefore a simple way of studying this type of modification is important.
Edward Tate and colleagues at Imperial College, London have done just this. They first modified cholesterol molecules to bear an azide group and then gave this to their target cells, where it was used in PTM. They next managed to attach, via ‘click’ chemistry, a dye molecule called TAMRA to the modified proteins that carried the synthetic cholesterol. The team used this dye for ‘fluorescence visualisation’ of the target protein.
When compared to traditional techniques for studying cholesterylated proteins, this new method stacks up well. It makes significant savings in both time and expense, as well as avoiding the use of potentially harmful radiation. Furthermore, Tate suspects that in the future an optimised version of this process might be used to search for new cholesterylated proteins.
Want to find out more? Then download the ChemComm article for free today. You can also check out coverage of this article in C&EN.