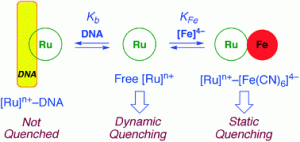
The binding of luminescent complexes to DNA is a popular area of research, with applications ranging from molecular switches to photodynamic therapy. It has commonly been reported in the literature that a lack of luminescence quenching by ferrocyanide, [Fe(CN)6]4−, can be used as evidence of the intercalation of a complex with DNA.
Claudia Turro and colleagues at Ohio State University have now shown that a Ru(II) complex that binds strongly to DNA electrostatically rather than by intercalation is equally resistant to 3MLCT (metal-to-ligand charge transfer) emission quenching as one known to be a DNA intercalator.
These findings indicate that the absence of emission quenching by [Fe(CN)6]4− cannot be used alone as proof of DNA intercalation by a complex. Hence, researchers using this method will need to take extra care when interpreting their results.
To find out more and start a discussion download the communication (for free until 18th Feb 2011) and leave your comments below.