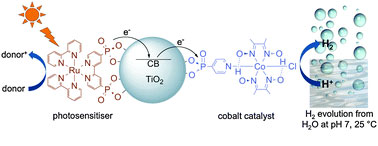
An inexpensive, easy to assemble light-activated water-splitting system for generating hydrogen has been devised by UK scientists.
Erwin Reisner at the University of Manchester* attached an inexpensive metal, cobalt, to ruthenium dye-sensitised titania nanoparticles. They placed the nanoparticles in water, added triethanolamine (which donates an electron), stirred the mixture at room temperature and found that hydrogen was generated.
Nanoparticles show excellent dispersibility in water and the high surface area allows for easy variation of the catalyst loading and ratio for the optimisation of light absorption and catalysis, says Reisner.
Download Reisner’s ChemComm communication to find out more. This article is part of the ChemComm Hydrogen web theme.
*now at the University of Cambridge