A hydrazine-based ionic liquid could be used as rocket fuel, say US scientists.
The combustibility of ionic liquids, a hazard for most applications, has inspired researchers to consider them as possible propellants in rocket fuels. Robin Rogers and his colleagues from The University of Alabama in Tuscaloosa and C3 Propulsion in Huntsville have found that an ionic liquid based on one of the most widely used rocket fuel propellants, hydrazine, ignited on contact with a catalyst, without the need for an oxidant or an ignition source.
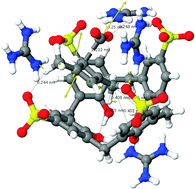
The team studied the behaviour of nitrate salts of 2-hydroxyethylhydrazinium upon addition of various catalysts. It was found that in the presence of one particular solid catalyst, Ir-alumina (known as Shell 405), and at temperatures above only 100 °C, the salts ignited, producing smoke and gas.

Addition of 2-hydroxyethylhydrazinium dinitrate to the solid catalyst Shell 405, followed by smoke, flame and residual catalyst
Rogers explains that the impetus for the research was increased safety: ‘we wanted to see if we could take a hydrazine-like molecule, which is volatile, turn it into a non-volatile salt and yet still have the same reactivity that the hydrazine did. We were pretty excited because it worked.’
In addition to decreasing vapour toxicity by replacing hydrazine with an ionic liquid, this material could lead to safer, more efficient, forms of rocket fuel since ignition could occur simply by running the ionic liquid over a catalyst bed, eliminating the need for stabilisers or an additional liquid oxidant.
‘Clearly, this is not only a safer way of propellant ignition, it is also more effective as it allows for low temperature combustion,’ says Christopher Hardacre, an expert in ionic liquids and catalysis from Queen’s University Belfast in Northern Ireland. Hardacre adds that the low volatility associated with ionic liquids as well as the low cost of the materials makes this propellant system even more attractive for various applications.
The work is still in its infancy and the next step will be to optimise the material for specific applications. ‘We need the real rocket scientists out there to weigh in and tell us what needs to be improved or to find better molecules or better salts,’ says Rogers.
Patricia Pantos
For more information, download Rogers’ ChemComm communication.