UK chemists have developed a new variation on a famous route to indoles that uses more readily available starting materials.
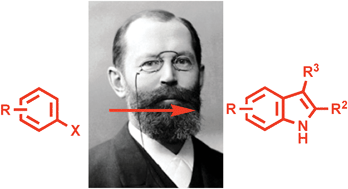
The Fischer reaction involves the functionalisation of an unactivated C-H position by way of a [3,3]-sigmatropic shift. It is simple and convenient – it couples a mono-functionalised arene with a readily available ketone or aldehyde – but is hindered by the lack of availability of aryl hydrazine starting materials.
Instead Christopher Moody and Martyn Inman at the University of Nottingham started from readily available haloarenes. They converted them into a wide range of indoles in just two steps by halogen-magnesium exchange, quenching with di-tert-butyl azodicarboxylate, then reacting with ketones under acidic conditions.
This new variation is simple and versatile, says Moody, making it a highly practical alternative modern protocol for making the fundamentally important indole ring system.
Download the article for free until 24th December and let us know what you think of this new route below.