A new type hypervalent iodine reagent could enable more efficient and green catalytic oxidations, report Japanese chemists.
Hypervalent iodine reagents have emerged as promising organo-oxidants, replacing classical heavy metal oxidants such as lead and mercury. m-Chloroperbenzoic acid is commonly used as a stoichiometric reoxidant to regenerate the reactive iodine species, but scientists would like to use other, more environmentally friendly oxidants, such as peracetic acid, instead. Until now, however, no efficient iodine catalyst that works well with peracetic acid has been found.
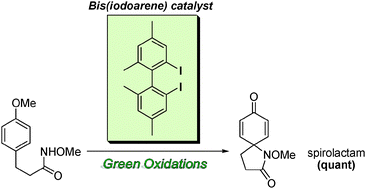
Yasuyuki Kita, at Ritsumeikan University, Kusatsu, and colleagues discovered that their µ-oxo-bridged hypervalent iodine(III) compounds, in the presence of peracetic acid, could catalyse spirolactam formation. They also believe the compounds can catalyse other types of oxidation, such as phenolic oxidation, and, because they have a chiral structure, could have potential in asymmetric oxidative transformations.
Download the communication for free until 5th November 2011. And if you have some exciting research of your own to report, submit your manuscript to ChemComm today.