Heterogeneous catalysis is widely used industrially to assist in the production of many man made materials. The world economy is reliant upon production of these materials, therefore catalysts which are reliable and cheap are hugely important. Heterogeneous catalysts generally fulfill these criteria, hence their wide application, but the understanding of how they function is often poorly defined.
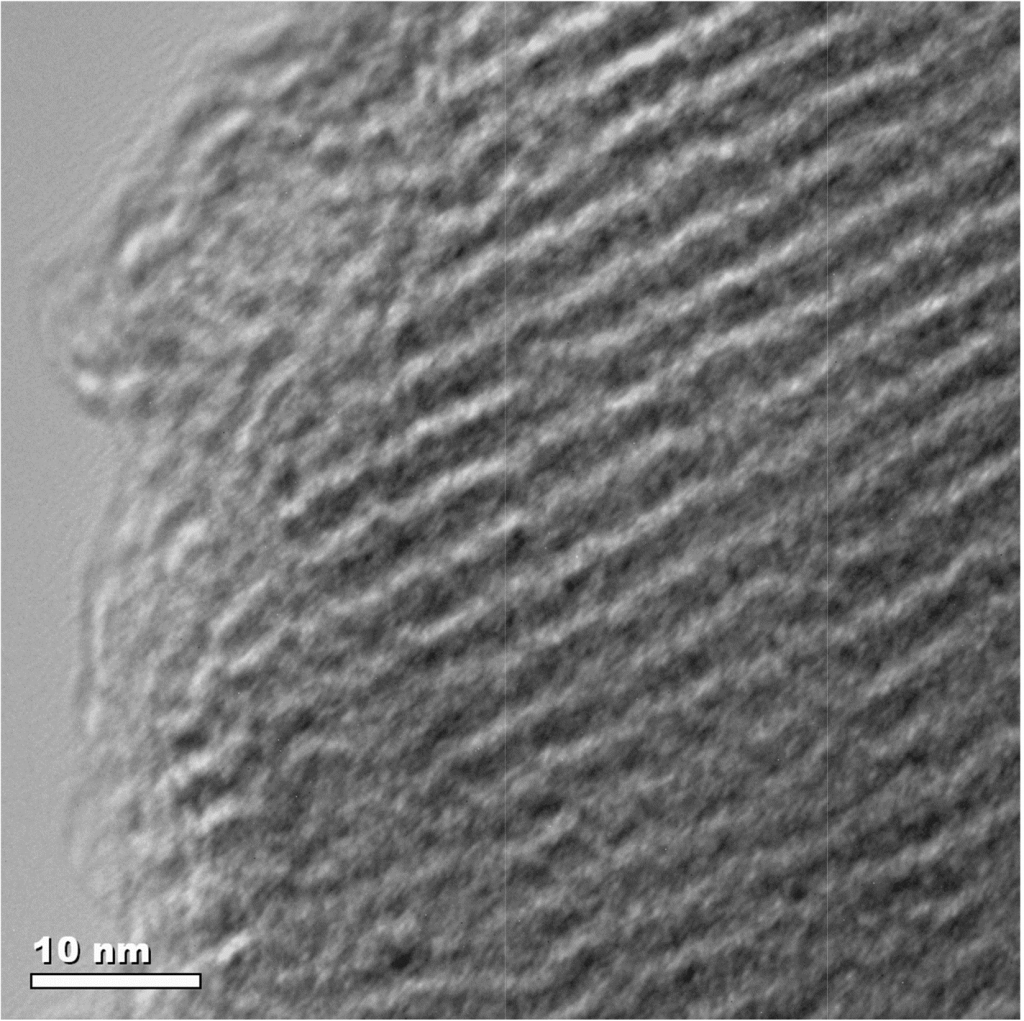 Academic investigations of single site homogeneous catalysts are generally simpler to study, which makes defining what reactions occurs at each stage and the factors which influence them clearer to interpret. Heterogeneous catalysts are more complex to study. The interaction between the different phases must be considered along with the nature of the catalyst itself. The catalysts are generally solid materials whose bulk composition may not provide an accurate picture of the surface where the reactions will ultimately be occurring and the activity may be quite different. Analysis of the subtleties of what is happening at the surface is incredibly difficult with many of the commonly used techniques not capable of providing the detail required. But in order to fully understand a heterogeneous catalyst it is essential to have an understanding of the how the separate phases interact.
Academic investigations of single site homogeneous catalysts are generally simpler to study, which makes defining what reactions occurs at each stage and the factors which influence them clearer to interpret. Heterogeneous catalysts are more complex to study. The interaction between the different phases must be considered along with the nature of the catalyst itself. The catalysts are generally solid materials whose bulk composition may not provide an accurate picture of the surface where the reactions will ultimately be occurring and the activity may be quite different. Analysis of the subtleties of what is happening at the surface is incredibly difficult with many of the commonly used techniques not capable of providing the detail required. But in order to fully understand a heterogeneous catalyst it is essential to have an understanding of the how the separate phases interact.
Tristan Youngs of the ISIS Facility, Christopher Hardacre of Queen’s University Belfast, and their co-workers have reported a new method to study a heterogenous process in real time. Neutron scattering experiments (which were only made possible by the state of the art facilities at ISIS) provide the ability to simultaneously examine the rate at which reactions occur and also the speed with which the different phases in a heterogeneous system can interact. A commonly held principle of heterogeneous catalysis is that by constructing pores and channels in the catalyst we can increase the effective surface area of the catalyst, increasing the number of sites where reactions can occur, thereby increasing the effectiveness of the catalysts.
This study, for the first time, highlights that the speed of the process is critically dependent on how easily the molecules can pass in and out of these pores. While this may not be the case for every process, challenging some of these commonly held beliefs will surely lead to a rethink of how catalysts are designed in the future.
Interested in more? Read this HOT, Open Access Chem Sci Edge article now!
Probing chemistry and kinetics of reactions in heterogeneous catalysts
Tristan G. A. Youngs, Haresh Manyar, Daniel T. Bowron, Lynn F. Gladden and Christopher Hardacre
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC51477C
Ruaraidh McIntosh is a guest web-writer for Chemical Science. His research interests include supramolecular chemistry and catalysis. When not working as a Research Fellow at Heriot-Watt University, Ruaraidh can usually be found in the kitchen where he has found a secondary application for his redoubtable skills in burning and profanity.










