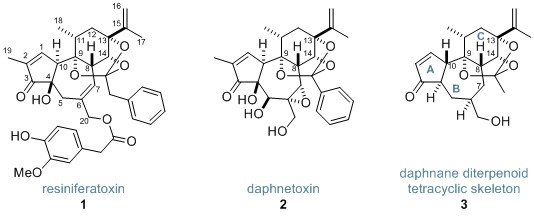
Resiniferatoxin (1) and daphnetoxin (2) are members of the daphnane diterpenoids—a class of diterpenoid orthoester compounds, many of which exhibit fascinating therapeutic activity. Resiniferatoxin, whose total synthesis has been achieved only once, is a strong analgesic possessing a complex and densely functionalised tetracyclic core. Now, the Inoue research group at the University of Tokyo have used a series of radical reactions to construct the tetracyclic skeleton of the daphnane diterpenoids (3).
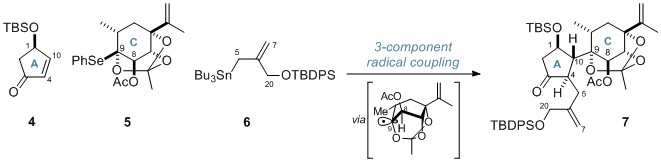
The first radical process was a 3-component coupling of a cyclopentanone (4), O,Se-acetal 5 and an allylstannane (6), and proceeded via a bridgehead radical generated from the reactive O,Se-acetal species. This impressive coupling assembled the A and C rings of the cyclic skeleton, forming 5 consecutive stereocentres in one step. These stereocentres were generated with high stereoselectivity with regards to the tertiary centres at C4 and C10, while the reaction was stereospecific for the creation of the tetrasubstituted C9 centre.
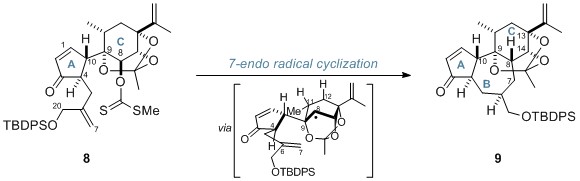
Subsequently, the researchers performed a 7-endo radical cyclisation of xanthate 8 in order to construct the 7-membered B ring. The protected daphnane diterpenoid skeleton (9) was produced as a single isomer with the desired stereochemistry at C8.
Tetracyclic skeleton 3 represents a common intermediate which, following functional group manipulations, would allow the synthesis of naturally-occurring daphnane diterpenoids, including resiniferatoxin, in addition to artificial analogues. The approach demonstrated by the Inoue group showcases the power of radical reactions in the formation of highly congested carbon frameworks and reinforces their relevance in the field of total synthesis.