The synthesis of tailor-made peptide chains represents a powerful tool for tuning the structure and properties of peptides, allowing for the development of analogues for medical, technological and synthetic purposes.
For example, the β-peptide is a synthetic peptide, which, in contrast to its naturally-occurring α-peptide analogue, is bonded through the β-carbon rather than the α-carbon. As a result of this seemingly small structural change, alterations in the peptide’s secondary structure and thermodynamic stability are observed.
Adding fluoride groups to peptide chains represents another way to alter and stabilise the folding structure through the presence of stronger hydrogen bonds and the introduction of fluorophilicity. This approach is generally employed for the addition of fluoride groups at ‘remote positions,’ spaced two or more methylene units from the peptide backbone. However, this method has less of an effect on the conformation of the peptide itself, and instead primarily influences the tertiary and quaternary self-aggregation of peptide chains, as a result of the fluorophilic effect of the functionalised peptide chains.
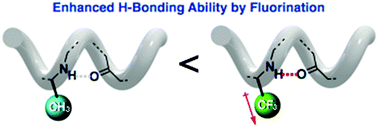
Much less commonly studied is the effect of incorporating fluorine groups in ‘direct proximity’ to the peptide chain, that is, directly attached to the β-carbon, where it is proposed that the intramolecular hydrogen bonding will be directly affected, and consequently, so too will the secondary structure of the peptide chain.
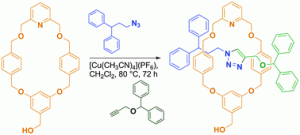
Yasuhiro Ishida and co-workers from the RIKEN Center for Emergent Matter Science have shown that this ‘direct’ fluorination of β-peptides can, in fact, affect the higher order structures of these peptide chains. Specifically, a hexameric β-peptide was designed, which consisted of cyclohexane-based β-amino acids in the 1-,3-,4- and 6-positions and L-alanine derivatives in the 2- and 5-positions, where the L-alanine methyl groups were either native or perfluorinated.
Irrespective of the degree of perfluorination in the β-peptide, it was found that the chains were arranged in the same left-handed 14-helix structure, with the NH-amide of the second and fifth residues participating in stabilising intramolecular H-bonding interactions. Moreover, it was found that although the presence of fluoride groups did not noticeably alter the overall secondary structure of the β-peptide chains, the stability of these structures was dramatically enhanced, showing the significant effect that fluoride groups can have on the hydrogen-bond donating ability of NH-amides.
This new approach of modifying peptide chains offers an interesting method for influencing the secondary, and higher order, structures of the compounds, as well as their kinetic and thermodynamic properties. The effect of these structural modifications offers the possibility of tuning the chemical and biological properties of these peptide chains for use in new types of antibiotics and synthetic systems.
Read this HOT ChemComm article in full!
Stabilization of β-peptide helices by direct attachment of trifluoromethyl groups to peptide backbones
Joonil Cho, Kyohei Sawaki, Shinya Hanashima, Yoshiki Yamaguchi, Motoo Shiro, Kazuhiko Saigo and Yasuhiro Ishida
Chem. Commun., 2014, 50, 9855–9858.
 About the Writer
About the Writer
Anthea Blackburn is a guest web writer for Chemical Communications. Anthea is a graduate student hailing from New Zealand, studying at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she is exploiting supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allow, she is ambitiously attempting to visit all 50 US states before graduation.














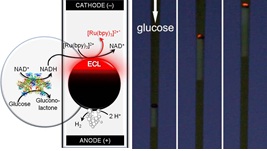
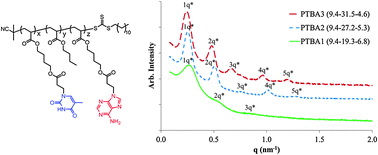
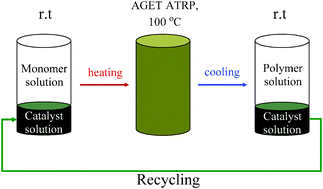
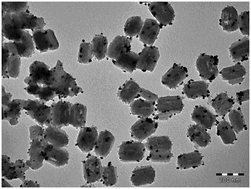
 Brass has been known to man since prehistoric times; now scientists in Germany have
Brass has been known to man since prehistoric times; now scientists in Germany have 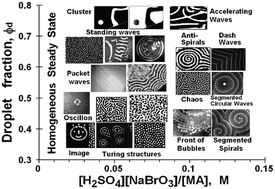
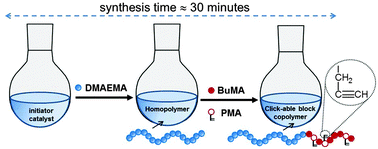
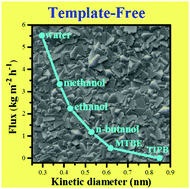
 A team in the US has shown that
A team in the US has shown that