A joint group of scientists from China, Japan and Australia recently made a breakthrough in water desalination. They designed and synthesized a multilayered electrode consisting of a graphene nanosheet sandwiched between two porous carbon particle layers. This “sandwich” electrode can be used for capacitive desalination to produce fresh water from seawater, and exhibited the highest desalination capacity among the reported graphene sheet-based electrodes.
Capacitive desalination is an emerging water desalination technique. It removes water-soluble salts, mostly sodium chloride, by applying an electric field to move the salts to the surface of electrodes. Because the amount of ions being removed is directly proportional to the surface area of the electrodes, using electrodes with abundant surface to electro-adsorb ions is critical for excellent desalination performance.
The researchers utilized graphene oxide (GO) and zeolitic imidazolate framework-8 (ZIF-8, a metal organic framework) as the two components (Figure 1a). When dissolved in water, ZIF-8 nanocrystals became attached to the surface of GO and completely covered both sides of the GO nanosheets. This process was driven by the coordination interaction between the two species. The formed ZIF-8/GO/ZIF-8 “sandwiches” were then annealed at near 1000 oC in nitrogen gas. The annealing step converted GO nanosheets and ZIF-8 nanocrystals into graphene nanosheets and porous carbon particle layers, respectively. Owing to the presence of pores on the surface of the yielded carbon particles, the carbon “sandwiches” had a high surface area of 1360 m2/g, much higher than that of the graphene sheets alone (150 m2/g).
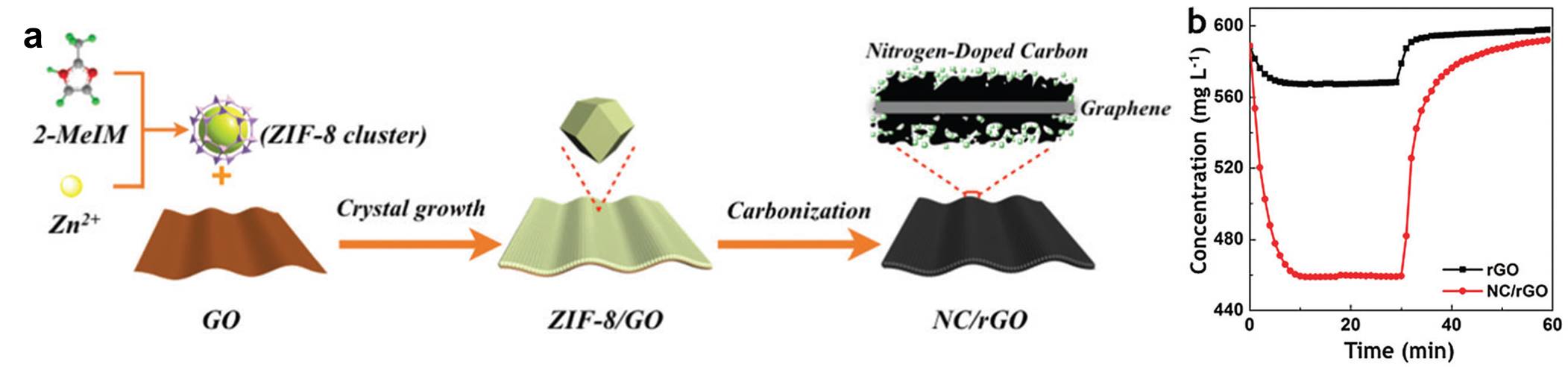
Figure 1. (a) A schematic diagram displaying the key steps for the synthesis of the carbon “sandwiched” electrodes. 2-MeIM = 2-methylimidazole, a building block for ZIF-8. (b) The change of NaCl concentration collected for a “sandwiched” electrode (NC/rGO) and a graphene sheet electrode (rGO). When an electric field is applied, the concentration of NaCl starts to drop and reaches a plateau; When the electric field dissipates, the concentration of NaCl returns to its initial level. The salt concentration decreased to a much lower level with NC/rGO (red curve) than rGO (black curve).
The desalination capacity of the carbon “sandwich” reaches 17.52 mg/g, meaning 1 gram of the electrode can remove 17.52 mg of sodium chloride. Consistent with the enhanced surface area, the capacity of the “sandwich” is much higher than that of the graphene alone (Figure 1b). More significantly, the “sandwich” electrode outperforms all other previously reported graphene sheet-based electrodes in terms of the desalination capacity.
This work has greatly advanced the development of capacitive desalination, a promising and affordable technique to mass produce fresh water by desalting seawater.
To find out more please read:
Miao Wang, Xingtao Xu, Jing Tang, Shujin Hou, Md. Shahriar A. Hossain, Likun Pan and Yusuke Yamauchi
Chem. Commun. 2017, 53, 10784-10787
About the blogger:
 Tianyu Liu is a Ph.D. in chemistry graduated from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web blogger for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu is a Ph.D. in chemistry graduated from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web blogger for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.










