Proteins are very useful molecules and when they work together, or assemble, they can display biocatalytic cascades, performing sequential multistep transformations of substrates. Scientists have tried to mimic nature for years, by creating artificial multi-enzyme complexes to replicate these biomolecules’ ability to catalyse reactions for use in biofuels, bioelectronics, bioproduction etc.
The arrangement of the proteins’ active sites relative to one another is intrinsic to the success of these reactions. One method of synthetically engineering these arrangements is through the use of DNA nanostructures. DNA aptamers can be used as scaffolds to encourage the proteins’ assembly and even introduce other functional properties – imagine this as the bottom layer of a human pyramid in Cirque du Soleil.
However, the DNA scaffolds are reported to degrade and the protein assemblies decompose. (Now, imagine someone telling a really good joke to the bottom layer of the human pyramid and it all falling apart.) The scaffolds and proteins are difficult to separate and this has limited the application of this strategy. Until now….
Masahiro Goto and co-workers have managed to arrange protein molecules (in this case, thrombin) on a DNA scaffold with the use of a DNA aptamers. With the addition of a chemical cross-linker, the neighbouring protein molecules were covalently cross-linked and retained their activity.
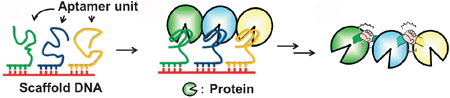
Using a DNA template for thrombin binding aptamers, and hybridising that with three thrombin binding aptamers with sticky ends, they formed a comb-like structure with branched arms. The thrombin molecules bind with these arms and a chemical cross-linker encourages the neighbouring thrombins to cross-link. This has been intonated on the diagram with ‘holding hands’. (Told you they were inspired by Cirque du Soleil).
Using polyacrylamide gel electrophoresis (PAGE), the group elegantly illustrated their results, successfully demonstrating that DNA scaffolds can produce successful protein-protein conjugation. The group continue to develop and improve their work to overcome limitations in the size of conjugate proteins, efficiency and applications.
Find out more – download the ChemComm communication, free for 4 weeks.