Looking back at 2019, we would like to highlight some of the great research that has been published in RSC Advances over the year. We are proud to present a selection of the most popular 2019 articles published on photocatalysis so far.
We hope you enjoy reading these articles – free to read and accessible to everyone.
Happy New Year from the RSC Advances team!
Strain-enhanced properties of van der Waals heterostructure based on blue phosphorus and g-GaN as a visible-light-driven photocatalyst for water splitting
Kai Ren, Sake Wang, Yi Luo, Yujing Xu, Minglei Sun, Jin Yu and Wencheng Tang
RSC Adv., 2019,9, 4816-4823. DOI: 10.1039/c8ra09378d
Photocatalytic dye degradation and biological activities of the Fe2O3/Cu2O nanocomposite
Mavinakere Ramesh Abhilash, Gangadhar Akshatha and Shivanna Srikantaswamy
RSC Adv., 2019,9, 8557-8568. DOI: 10.1039/c8ra09929d
Isolated/interacting Au islands on TiO2 NTs for the switching photocatalytic/photoelectrocatalytic degradation of refractory organic pollutants in wastewater
Dan Zhang, Baohui Wang, Jiaqi Wang, Hongming Wang, Shixu Zhang and Di Gu
RSC Adv., 2019,9, 2784-2791. DOI: 10.1039/c8ra09160a
ZnO decorated Sn3O4 nanosheet nano-heterostructure: a stable photocatalyst for water splitting and dye degradation under natural sunlight
Sagar D. Balgude, Yogesh A. Sethi, Bharat B. Kale, Dinesh P. Amalnerkar and Parag V. Adhyapak
RSC Adv., 2019,9, 10289-10296. DOI: 10.1039/c9ra00788a
Fabrication of interlayer beta-CD/g-C3N4@MoS2 for highly enhanced photodegradation of glyphosate under simulated sunlight irradiation
Xiufang He, Zhansheng Wu, Yongtao Xue, Zhenzhen Gao and Xia Yang
RSC Adv., 2019,9, 4635-4643. DOI: 10.1039/c8ra10190f
Multi-shelled ZnO decorated with nitrogen and phosphorus co-doped carbon quantum dots: synthesis and enhanced photodegradation activity of methylene blue in aqueous solutions
Shaojia Song, Kun Wu, Huadong Wu, Jia Guo and Linfeng Zhang
RSC Adv., 2019,9, 7362-7374. DOI: 10.1039/c9ra00168a
A Bi2WO6/Ag2S/ZnS Z-scheme heterojunction photocatalyst with enhanced visible-light photoactivity towards the degradation of multiple dye pollutants
Soleiman Mosleh, Kheibar Dashtian, Mehrorang Ghaedi and Maryam Amiri
RSC Adv., 2019,9, 30100-30111. DOI: 10.1039/c9ra05372g
Visible-light photocatalytic performance, recovery and degradation mechanism of ternary magnetic Fe3O4/BiOBr/BiOI composite
Jianhui Li, Fan Yang, Quan Zhou, Lijie Wu, Wenying Li, Ruipeng Ren and Yongkang Lv
RSC Adv., 2019,9, 23545-23553. DOI: 10.1039/c9ra04412d
Engineering a CsPbBr3-based nanocomposite for efficient photocatalytic CO2 reduction: improved charge separation concomitant with increased activity sites
Xiao-Xuan Guo, Shang-Feng Tang, Yan-Fei Mu, Li-Yuan Wu, Guang-Xing Dong and Min Zhang
RSC Adv., 2019,9, 34342-34348. DOI: 10.1039/c9ra07236e
TiO2 nanocrystals with the {001} and {101} facets co-exposed with MIL-100(Fe): an egg-like composite nanomaterial for efficient visible light-driven photocatalysis
Wan Wu, Jie Zhu, Yue Hong Deng, Ye Xiang, Ya Wen Tan, Hai Qin Tang, Hao Zou, Yi Feng Xu and Yi Zhou
RSC Adv., 2019,9, 31728-31734. DOI: 10.1039/c9ra06359e
Light-controlled two-dimensional TiO2 plate micromotors
Ying Wang, Zhen Li, Alexander A. Solovev, Gaoshan Huang and Yongfeng Mei
RSC Adv., 2019,9, 29433-29439. DOI: 10.1039/c9ra06426e
Facile synthesis of few-layer MoS2 in MgAl-LDH layers for enhanced visible-light photocatalytic activity
Guoyuan Zheng, Caihong Wu, Jilin Wang, Shuyi Mo, Yanwu Wang, Zhengguang Zou, Bing Zhou and Fei Long
RSC Adv., 2019,9, 24280-24290. DOI: 10.1039/c9ra03858b

Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.












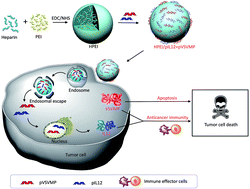
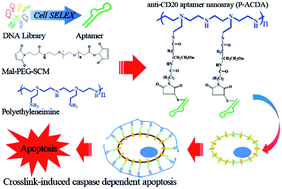
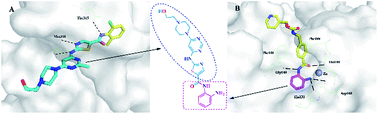 To determine the effect of the hybrid drugs on cancer cell survival, the research team tested the drug’s ability to halt the growth of three cell types exhibiting features of leukemia, kidney cancer and prostate cancer respectively. They found that all drugs in the series were toxic to cancer cells, with leukemia and kidney cancer cells showing the greatest degree of sensitivity to the hybrid drugs.
To determine the effect of the hybrid drugs on cancer cell survival, the research team tested the drug’s ability to halt the growth of three cell types exhibiting features of leukemia, kidney cancer and prostate cancer respectively. They found that all drugs in the series were toxic to cancer cells, with leukemia and kidney cancer cells showing the greatest degree of sensitivity to the hybrid drugs.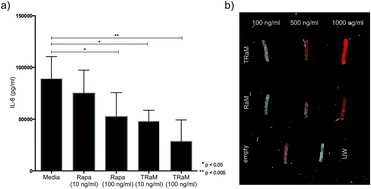 Their studies showed that the rapamycin-loaded nanoparticles were stable and biocompatible when tested in human endothelial cells. Further, the rapamycin release could be controlled by adjusting the pH values lower than 7 or higher than 8. The study found that the micelles were being taken up by cells within 6h after incubation. The study also demonstrates the specificity of the micelles by showing that what the cells were pre-treated with an integrin inhibitor, they were less likely to take up the micelles.
Their studies showed that the rapamycin-loaded nanoparticles were stable and biocompatible when tested in human endothelial cells. Further, the rapamycin release could be controlled by adjusting the pH values lower than 7 or higher than 8. The study found that the micelles were being taken up by cells within 6h after incubation. The study also demonstrates the specificity of the micelles by showing that what the cells were pre-treated with an integrin inhibitor, they were less likely to take up the micelles.