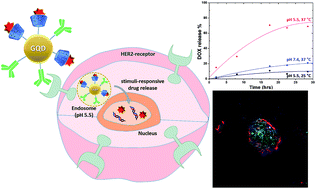
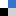Looking back at 2019, we would like to highlight some of the great research that has been published in RSC Advances over the year. We are proud to present a selection of the most popular 2019 articles published on organic synthesis so far.
We hope you enjoy reading these articles – free to read and accessible to everyone.
Happy New Year from the RSC Advances team!
Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N-H compounds
Sattar Arshadi, Saeideh Ebrahimiasl, Akram Hosseinian, Aazam Monfared and Esmail Vessally
RSC Adv., 2019,9, 8964-8976. DOI: 10.1039/c9ra00929a
Review
Nitroketene N,S-acetals: synergistic building blocks for the synthesis of heterocycles
Saigal, Sarfaraz Khan, Habibur Rahman, Shafiullah and Md. Musawwer Khan
RSC Adv., 2019,9, 14477-14502. DOI: 10.1039/c9ra00630c
Review
Pyrazolo-fused 4-azafluorenones as key reagents for the synthesis of fluorescent dicyanovinylidene-substituted derivatives
Jessica Orrego-Hernández, Carolina Lizarazo, Justo Cobo and Jaime Portilla
RSC Adv., 2019,9, 27318-27323. DOI: 10.1039/c9ra04682h
Impact of an aryl bulky group on a one-pot reaction of aldehyde with malononitrile and N-substituted 2-cyanoacetamide
Ruturajsinh M. Vala, Divyang M. Patel, Mayank G. Sharma and Hitendra M. Patel
RSC Adv., 2019,9, 28886-28893. DOI: 10.1039/c9ra05975j
Continuous-flow synthesis of 3,5-disubstituted pyrazoles via sequential alkyne homocoupling and Cope-type hydroamination
Sándor B. Ötvös, Ádám Georgiádes, Dániel Ozsvár and Ferenc Fülöp
RSC Adv., 2019,9, 8197-8203. DOI: 10.1039/c9ra01590f
Synthesis and biological activities of petrosiols B and D
Jialin Geng, Qidong Ren, Caizhu Chang, Xinni Xie, Jun Liu and Yuguo Du
RSC Adv., 2019,9, 10253-10263. DOI: 10.1039/c9ra01166h
Synthesis of d-glyco-alkynone derivatives via carbonylative Sonogashira reaction
Mariana P. Darbem, C. Henrique A. Esteves, Isadora M. de Oliveira, Joel S. Reis, Daniel C. Pimenta and Hélio A. Stefani
RSC Adv., 2019,9, 9468-9474. DOI: 10.1039/c9ra00523d
A facile approach to 2-alkoxyindolin-3-one and its application to the synthesis of N-benzyl matemone
Makoto Shimizu, Hayao Imazato, Isao Mizota and Yusong Zhu
RSC Adv., 2019,9, 17341-17346. DOI: 10.1039/c9ra02204j
Merging catalyst-free synthesis and iodine catalysis: one-pot synthesis of dihydrofuropyrimidines and spirodihydrofuropyrimidine pyrazolones
Ya-Yun Zheng, Kai-Xiang Feng, Ai-Bao Xia, Jie Liu, Cheng-Ke Tang, Zhan-Yu Zhou and Dan-Qian Xu
RSC Adv., 2019,9, 9770-9776. DOI: 10.1039/c9ra01665a
Enantioselective synthesis of anti-3-alkenyl-2-amido-3-hydroxy esters: application to the total synthesis of (+)-alexine
Lu Yu and Peter Somfai
RSC Adv., 2019,9, 2799-2802. DOI: 10.1039/c9ra00173e
1-Alkyl-3-alkylindolin-2-imine hydrochlorides as useful building blocks in the copper-catalyzed synthesis of polycyclic indoline scaffolds
Can Liu, Haijun Yang, Changjin Zhu and Hua Fu
RSC Adv., 2019,9, 8369-8372. DOI: 10.1039/c9ra00995g
Microwave-assisted organic synthesis of nucleoside ProTide analogues
Cinzia Bordoni, Cecilia Maria Cima, Elisa Azzali, Gabriele Costantino and Andrea Brancale
RSC Adv., 2019,9, 20113-20117. DOI: 10.1039/c9ra01754b
Cap control: cyclic versus linear oligomerisation in covalent template-directed synthesis
Diego Núñez-Villanueva, Maria Ciaccia and Christopher A. Hunter
RSC Adv., 2019,9, 29566-29569. DOI: 10.1039/c9ra07233k
Copper-catalysed enantioselective intramolecular etherification of propargylic esters: synthetic approach to chiral isochromans
Shiyao Liu, Kazunari Nakajima and Yoshiaki Nishibayashi
RSC Adv., 2019,9, 18918-18922. DOI: 10.1039/c9ra03880a
Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds
Aref Mohammadi, Mohammad Bayat and Shima Nasri
RSC Adv., 2019,9, 16525-16533. DOI: 10.1039/c9ra03214b
Aggregation-induced chiroptical generation and photoinduced switching of achiral azobenzene-alt-fluorene copolymer endowed with left- and right-handed helical polysilanes
Hailing Chen, Lu Yin, Meng Liu, Laibing Wang, Michiya Fujiki, Wei Zhang and Xiulin Zhu
RSC Adv., 2019,9, 4849-4856. DOI: 10.1039/c8ra09345h
Palladium-catalyzed oxidative cross-coupling for the synthesis of α-amino ketones
Xiao-Hong Wei, Zhen-Hua Li, Lian-Biao Zhao, Ping Zhang, Han-Cheng Zhou and Yan-Bin Wang
RSC Adv., 2019,9, 32081-32084. DOI: 10.1039/c9ra06108h

Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.