We are very pleased to introduce Professor Ponnadurai Ramasami, who is joint corresponding author on the paper, Theoretical study of a derivative of chlorophosphine with aliphatic and aromatic Grignard reagents: SN2@P or the novel SN2@Cl followed by SN2@C?. The manuscript was well received by reviewers and was handpicked by our reviewers and handling editors to be part of our Popular Advances collection. Ponnadurai told us more about the work that went into this article and what he hopes to achieve in the future. You can find out more about the authors and their article below. To view our other Popular Advances, please explore our collection here.

Professor Ponnadurai Ramasami, CSci, CChem, FRSC, FICCE, MMast, received his PhD in Physical Chemistry and became full Professor in 2013. He leads the Computational Chemistry Group, Department of Chemistry, Faculty of Science at the University of Mauritius. The research group focuses on the use of computational methods to solve chemistry and interdisciplinary problems. The group is particularly interested in collaborating with experimentalists, and they use computational methods to complement experimental research. He has already published 260 research papers in peer-reviewed journals and he has edited several books. He is the chairman of the annual Virtual Conference on Chemistry and its Applications.
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
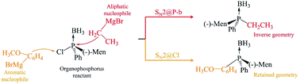
The focus of the article is the computational investigation of SN2 reactions in organic molecules which contain both phosphorus and chlorine atoms.
The SN2 reaction mechanism was discovered in the 1930’s by scientists Hughes and Ingold, and since then has been used in a number of syntheses; however, it is still of current interest as new aspects of this mechanism, at the molecular level, are still being discovered. These aspects include new sites of nucleophilic attack which are not immediately chemically intuitive.
How big an impact could your results potentially have?
In textbooks, SN2 reactions are defined in a firm way, often taking the example of SN2 at the carbon atom, detailing hill-shaped potential energy surfaces and nucleophilic attack at one specific atom centre. However, our research indicates that these well-established facts may change. Potential energy surfaces may take the shape of single, double or triple wells or a combination of hill and well shapes. The most preferred site of nucleophilic attack may change according to what neighbouring groups are present in the molecule of interest. It is important to include and try to explain these differences in chemistry textbooks.
Could you explain the motivation behind this study?
The Computational Chemistry Group of the University of Mauritius (CCUoM) was set up in 2003 in the Department of Chemistry. Our interest has always been on the investigation of different aspects of reaction mechanisms. We have a programme to study SN2 reaction mechanisms, which resulted in two PhD graduates and several publications. We started by studying the effect of different nucleophiles. Another part of the programme involved studying SN2 reactions at different atoms within one molecule. This started in 2017, when we came across one experimental study which involved SN2 at the phosphorus atom. We tried to explain the results of this experimental study using computational methods, which led us to discover SN2 at the chlorine atom.
In your opinion, what are the key design considerations for your study?
For SN2 reactions, the key design considerations involve the reactive atom centres, neighbouring groups, the solvent and the nucleophiles. These may be used to tune reactions to design molecules of interest.
Which part of the work towards this paper proved to be most challenging?
Working with bulky molecules was the most challenging part. Computations involving bulky molecules are demanding in terms of computational cost. It is often challenging to strike the right balance between computational cost and accuracy of results.
What aspect of your work are you most excited about at the moment?
When this research project started, it was about SN2 reactions at the phosphorus atom but along the research journey, we stumbled on the SN2 at the chlorine atom, which offers a new world of possibilities to investigate. The possibilities are what we are most excited about.
What is the next step? What work is planned?
Our next projects will involve changing key factors in the SN2 reaction mechanism involving the chlorine atom and determining the effect. We are considering changing the nucleophiles which we investigated, modifying the solvent system, and changing neighbouring groups. We are also considering investigating SN2 reactions at other reactive atoms, such as bromine and iodine.
Theoretical study of a derivative of chlorophosphine with aliphatic and aromatic Grignard reagents: SN2@P or the novel SN2@Cl followed by SN2@C?
Nandini Savoo,a Lydia Rhyman*ab and Ponnadurai Ramasami*ab

Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest Popular Advances articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.














