Scientists in the area of cancer drug development constantly find ways to target cancer’s Achilles heel. Bcr-Abl is a protein expressed in 95% of all Chronic Myelogenous Leukemia (a type of blood cancer) cases. It remains activated (i.e. switched ON) and instructs cancer cells to divide indefinitely. Bcr-Abl has remained an attractive target for therapy. Yet another attractive therapeutic target is Histone Deacetylase 1 (HDAC1) – a protein known to control cell survival via its ability to influence the turning on and turning off of certain genes.
Previous studies show that the drugs Dasatinib and MS-275 efficiently inhibit the cancer promoting activities of Bcr-Abl and HDAC1 respectively. Clinical trials also suggest that these drugs, when used independently in separate studies, can be used to treat a variety of solid as well as blood-borne cancers. Bcr-Abl and HDAC1 are components of distinct cellular wiring systems, referred to as signalling pathways, which sustain cell survival and division. Single agent drugs, or drugs that stifle a single cancer-promoting pathway, weed out most cancer cells but also set the stage for drug-resistant cells. Reports suggest that both Dasatinib and MS-275 are associated with cancer drug resistance.
Multi-target inhibitors are a new and evolving class of cancer drugs that can simultaneously inhibit at least two signalling pathways. These compounds have emerged as a potential solution in circumventing cancer drug resistance. Chen and colleagues at the Department of Organic Chemistry, School of Science, China Pharmaceutical University, China, designed and produced a series of hybrid drug molecules which combine the attributes of the HDAC inhibitor MS-275 with the Bcr-Abl inhibitor Dasatinib.
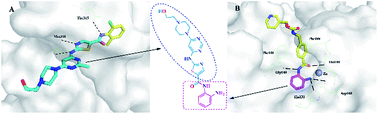
To better understand how the hybrid drugs interacted with the Bcr-Abl and HDAC1 active sites (i.e. the ON switch), the team relied on computer-generated three-dimensional models of the hybrid drugs, Bcr-Abl and HDAC1 proteins. Using a method similar to finding the right key for a lock, a computer program found that a hybrid compound termed 6a, which happened to be the most potent compound in the series, fit most snugly into both Bcr-Abl and HDAC1 active sites. In theory, 6a would prevent both Bcr-Abl and HDAC1 from becoming activated (i.e they remain switched OFF).
On the basis of these observations, this study strengthens the paradigm that chemically melding two cancer drugs to form a novel single molecule may prove to be an effective clinical strategy for anticancer treatment. On a broader scale, this is one among many studies advocating for the use of multi-target agents in cancer treatment, highlighting an imminent upsurge in single molecule combination therapies.
Read the full article here:










