 According to an estimate by the World Cancer Report, cancer associated mortality is expected to reach 17 million per year globally by the year 2030. To confront the cancer burden with appropriate clinical interventions, researchers screen cancer-killing drug combinations in monolayer cell cultures. This is a widely used method for the preclinical evaluation of drug efficacy.
According to an estimate by the World Cancer Report, cancer associated mortality is expected to reach 17 million per year globally by the year 2030. To confront the cancer burden with appropriate clinical interventions, researchers screen cancer-killing drug combinations in monolayer cell cultures. This is a widely used method for the preclinical evaluation of drug efficacy.
A major limitation of monolayer cultures is that they do not, even mildly, recapitulate the complex architecture of a tumor growing in vivo. As an initial step in overcoming this limitation, researchers use scaffolded spheroid cultures – a system wherein cells are grown on hydrogel scaffolds in three dimensions.
Hydrogel scaffolds provide physical and structural support for the formation of a more ‘natural’ setting that better recapitulates cell behavior in vivo. For example, smaller (150um) spheroids have better cell-to-cell contacts and notably different gene expression compared to monolayer cultures; larger (200-500um) ones develop oxygen and nutrient gradients reminiscent of chemical gradients seen in human tumors. However, limitations in design flexibility, handling and interbatch compositional variation have discouraged the routine use of hydrogel scaffolds. In addition, the technical challenge of separating newly formed spheroids from the scaffolding material before drug screening represents a major roadblock.
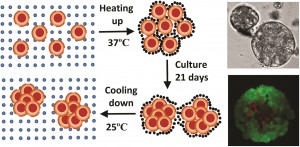
In a study led by Xiaolin Cui and colleagues at the School of Chemical Engineering and the School of Mathematical Sciences, University of Adelaide, Australia, researchers synthesized a thermo-reversible N-Isopropylacrylamide-acrylic acid (NIPAM-AA) hydrogel by free radical emulsion polymerization. In their study using the cervical carcinoma cell line HeLa, the team demonstrates that at 370C the NIPAM-AA hydrogel solidifies and forms a sheath around HeLa cell clusters. As a consequence, these clusters develop into hydrogel scaffolded spheroids over time. At 250C the hydrogel liquefies and releases the newly formed spheroids.
Cell viability assays confirmed that this new state of the art hydrogel is biocompatible. NIPAM-AA derived spheroids were smaller (70-120um), nearly spherical and showed a narrower size distribution compared to conventional spheroids. The study showed, through time course experiments, that the spheroids remain viable for over 14 days in culture. The study also suggests that spheroids derived via the NIPAM-AA hydrogel method are more viable than those derived from conventional suspension cultures, supporting the notion that hydrogel scaffolding facilitates oxygen and nutrient supply to support cell growth.
The researchers deduced a mathematical model to predict the kinetics of NIPAM-AA derived spheroid growth. Their model accurately recapitulated the growth rate, size and size distribution of the spheroids. The authors propose that hydrogel scaffolding has the potential to evolve into a technology with a wide range of applications including, but not limited to, (1) high throughput screening of anticancer drugs using uniformly sized spheroids; (2) regenerative medicine; and (3) tissue engineering.
Read the full article here:










