We are very pleased to introduce Magne Olav Sydnes, corresponding author of the paper ‘Photodegradable antimicrobial agents − synthesis, photodegradation, and biological evaluation‘. His article has been very well received and handpicked by our reviewers and handling editors as one of our HOT articles. Magne told us more about the work that went into this article and what he hopes to achieve in the future. You can find out more about the author and his article below and find more HOT articles in our online collection.
Meet the author
 Sydnes obtained his PhD degree in 2004 from Australian National University under the guidance of Professor Banwell. He then worked as a postdoctoral fellow both in Australia and Japan, including two years as a JSPS postdoctoral fellow in Professor Isobe’s group at Nagoya University. In 2009 he joined International Research Institute of Stavanger, Norway, as a researcher. Since December 2011 he has been at University of Stavanger. Research interests include natural product synthesis, medicinal chemistry, catalysis, chemical biology, analytical chemistry, and environmental chemistry.
Sydnes obtained his PhD degree in 2004 from Australian National University under the guidance of Professor Banwell. He then worked as a postdoctoral fellow both in Australia and Japan, including two years as a JSPS postdoctoral fellow in Professor Isobe’s group at Nagoya University. In 2009 he joined International Research Institute of Stavanger, Norway, as a researcher. Since December 2011 he has been at University of Stavanger. Research interests include natural product synthesis, medicinal chemistry, catalysis, chemical biology, analytical chemistry, and environmental chemistry.
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
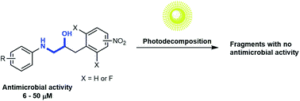
The focus of the article is to make compounds that have antimicrobial activity that can decompose under light. The long term aim of our work is to make antibiotics that can decompose in the environment after they have done their work in the patient (human or animal).
How big an impact could your results potentially have?
Antimicrobial resistance is a big and growing problem. We see this as a potential solution to this problem (one of several). With an antibiotic that decomposes after use it will not be laying around in the environment for long enough to make it possible for microorganisms to generate resistance towards it.
Could you explain the motivation behind this study?
The motivation behind this study was to establish a strategy that makes it possible to decompose the active compound into inactive fragments.
In your opinion, what are the key design considerations for your study?
Two key design concepts: 1) a new scaffold that has not been used in antibiotics previously; and 2) having a system that can decompose at a pH similar to the pH of natural water. One of our compounds decomposes very efficiently at pH 8 and the pH of sea water is 7.5-8.4.
Which part of the work towards this paper proved to be most challenging?
The method for how to make our compounds decompose under light we established quite early in the project. What was more troublesome was to make compounds that actually had biological activity.
What aspect of your work are you most excited about at the moment?
I am excited about all the possibilities that this chemistry opens. Not just for antibiotics but for pharmaceuticals in general. We do see an increasing concentration of a range of pharmaceuticals in the environment (including antibiotics). This chemistry opens the possibility to increase their decomposition after passing through the patient’s body.
What is the next step? What work is planned?
We plan to use the results in order to make more active compounds and also make compounds that has activity towards Gram-negative bacteria.
Photodegradable antimicrobial agents − synthesis, photodegradation, and biological evaluation
Vebjørn Eikemo, Leiv K. Sydnes and Magne O. Sydnes
RSC Adv., 2021,11, 32339-32345
DOI: 10.1039/D1RA06324C, Paper
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.