The continuous depletion of limited reserved fossil fuels and corresponding environmental concerns due to their combustion strictly demands the exploration of alternative energy resources for sustainability. Although solar and wind energies are harvested, the seasonal intermittence restricts their broad application. In this regard, the scientific community has developed the fuel cell using gaseous fuels like oxygen (at the cathode half-cell) and hydrogen (at the anode half-cell) that can produce electricity with better specific energies without compromising efficiency. The scalable production of these gaseous fuels (i.e. H2 and O2) has become a great challenge for energy researchers. Among the many processes and technologies developed, a green process for the production of these molecular fuels is the electrochemical splitting of water in an electrolyzer. The smooth running of the electrolyzer depends on both the cathodic and anodic half reactions. Whereas the cathodic half reaction (hydrogen evolution reaction; HER) is very straight forward, the multiproton-coupled electron transfer steps cause OER to face sluggish reaction kinetics demanding additional potential (overpotential) to overcome the reaction barrier. Hence, the HER is greatly hampered and thereby the overall electrolysis process. Since the efficiency of the half-cell strictly adheres on the catalytic efficacy of the electrocatalyst, researchers are focusing on the design of new catalyst materials. Among them, the transition metal based dichalcogenides (TMDs) and phosphides (TMPs) are the recent topics of study. Although these electrocatalysts catalyze OER efficiently, phase and composition changes during the course of reaction raises questions about the catalytically active centers of the electrocatalysts.
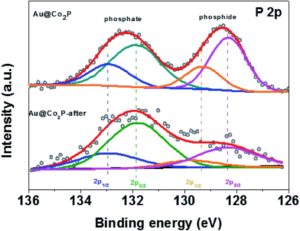
The in-depth characterization of post catalytic sample as well as in situ sample analysis clearly demonstrates the surface transformation of the TMDs and TMPs. In a particular study by Dutta and Samantara et al. have demonstrated the OER performances of Co2P nano needles in alkaline electrolytic conditions (1). As per the report, a significant broad peroxidation peak was observed in the linear sweep voltammetry signifying the surface oxidation of Co2P to corresponding oxides (CoOx). The interface of Co2P-CoOx facilitate carrier transportation from the core Co2P to oxides on the surface, thereby improving the electrocatalytic performances. Likewise, the core-shell Au@Co2P nanostructures derived via the wet chemical synthesis method were found to act as precursor catalysts for OER. However, the surface oxidized forms, i.e. Co-phosphates and Co-oxides/hydroxides, act as the real active centers of the electrocatalysts in alkaline conditions. The surface transformations were monitored by the X-ray photoelectron study of the post OER sample (2).
Similar surface transformations have been noticed also in case of TMDs. During the course of the OER, the surfaces of metal sulphides and selenides transform to their corresponding oxides and oxy-hydroxides and perform as active electrocatalysts to catalyze the water oxidation. Moreover, these surface transformed oxidized functionalities are more catalytically active than the parent TMDs, TMPs and the respective oxides alone. It is therefore imperative to characterize and define the real active centers of the catalysts used for water oxidation, particularly in alkaline electrolytic conditions.
References
- Anirban Dutta, Aneeya K. Samantara, Sumit K. Dutta, Bikash Kumar Jena, and Narayan Pradhan, ACS Energy Lett., 2016, 1, 1, 169–174.
- Xiaofang Zhang, Aixian Shan, Sibin Duan, Haofei Zhao, Rongming Wang and Woon-Ming Lau, RSC Adv., 2019,9, 40811-40818.
About the Web Writer:
Dr. Aneeya K. Samantara is Doctor in Chemical Sciences and currently has a Postdoctoral position (NPDF) in the School of Chemical Sciences, National Institute of Science Education and Research, Bhubaneswar, Odisha, India. Recently he joined as Community Board Member of the “Materials Horizon” of Royal Society of Chemistry, London. He pursued his PhD at the CSIR-Institute of Minerals and Materials Technology, Odisha, India. Before joining the PhD program, he completed his master of philosophy in chemistry at Utkal University and master in science in advanced organic chemistry at Ravenshaw University, Cuttack, Odisha, India. Dr. Samantara’s research interests include the synthesis of transition metal based electrocatalysts and graphene composites for energy storage and conversion applications. You can find him on Twitter at @cmrjitu.
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.