We are very pleased to introduce Atmika Paudel and Kazuhisa Sekimizu, authors of the paper ‘GPI0363 inhibits the interaction of RNA polymerase with DNA in Staphylococcus aureus’. Their article has been very well received and handpicked by our reviewers and handling editors as one of our HOT articles. Atmika and Kazuhisa were kind enough to tell us more about the work that went into this article and what they hope to achieve in the future. You can find out more about the authors and their article below and find more HOT articles in our online collection.
Meet the Authors

Dr Atmika Paudel received her undergraduate degree in Pharmacy in 2005 from Tribhuvan University in Nepal and went on to complete a Masters and PhD in Pharmaceutical Biology under the supervision of Professor Kazuhisa Sekimizu from the University of Tokyo in 2010 and 2013, respectively. Since graduation, Dr. Paudel has been a Research Fellow at the University of Tokyo and Teikyo University in Japan. Her research interest includes the discovery of novel therapeutically active antimicrobial agents active against drug-resistant superbugs using the silkworm infection model.

Professor Kazuhisa Sekimizu received his PhD in 1979 from the University of Tokyo by under the supervision of Professor Den’ichi Mizuno and is currently serving as the professor and director of Teikyo University Institute of Medical Mycology Japan. In his early years, Professor Sekimizu studied RNA polymerase and transcription elongation factor S-II in mammalian cells under the supervision of Professor Shunji Natori and initiation of DNA replication in Escherichia coli under the supervision of Professor Arthur Kornberg. His current research interest includes the development of silkworm as an animal model for the identification of therapeutic drugs and functional foods.
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
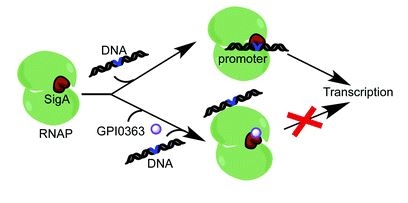
SigA, an essential enzyme required for bacterial transcription, does not exist in human beings and can be targeted for the development of antibiotics that specifically inhibit microbial growth. In this study, we found that GPI0363, a SigA binding antibiotic, inhibits transcription in a different manner compared to other transcription inhibitors recently in clinical use.
How big an impact could your results potentially have?
Microorganisms regularly acquire resistance against antibiotics used in the clinic. To overcome this problem, we should be ready to provide another antibiotic with a novel mode of action and a narrow spectrum of activity. The activity of GPI0363 against drug-resistant Staphylococcus aureus will allow us to develop this molecule as a therapeutic approach against drug-resistant pathogens.
Could you explain the motivation behind this study?
GPI0363 was identified in our laboratory by using the silkworm infection model in 2017. In our earlier study, we found that a single mutation in SigA was responsible for resistance to this antibiotic. This suggested that SigA can be a druggable target for antimicrobial agents and GPI0363 could be a new kind of SigA inhibitor. Thus, we were intrigued to study the underlying mechanism of the antistaphylococcal action of GPI0363. In this study, we explain the proof of concept for the utilization of SigA as a target of antimicrobial agents.
In your opinion, what are the key design considerations for your study?
Due to several problems associated with absorption, distribution, metabolism, excretion, and toxicity (ADMET), most of the antimicrobial agents that display activity in vitro do not show the in vivo activity. For this reason, we use silkworms at the beginning of screening so that the compounds that do not display therapeutic activity can quickly be discarded at the early stage.
In your article you mention that GPI0363 can serve as a promising lead molecule to develop staphylococcal RNA polymerase inhibitors. Please could you tell us more about this?
SigA is present in bacteria and differs among bacterial species. Our findings suggest that GPI0363 is selective towards the inhibition of staphylococcal RNA polymerase via SigA, thus can be used for the development of tailor-made medicines for the same. Further studies in GPI0363 through the structure-activity relationship study should lead to the discovery of compounds with more potent inhibitory activity and better therapeutic activity.
Which part of the work towards this paper proved to be most challenging?
The experiment to prove which step of transcription is inhibited was the most challenging to us. Our purified RNA polymerase fraction of Staphylococcus aureus contained a small amount of SigA and it was difficult to identify if GPI0363 inhibited the interaction of SigA with RNA polymerase core enzyme. To overcome this issue, we used Escherichia coli RNA polymerase core enzyme and S. aureus SigA to prepare a hybrid RNA polymerase holoenzyme.
What aspect of your work are you most excited about at the moment?
At this moment, I am excited for two reasons:
a. GPI0363 does not harbor cross-resistance against clinically used inhibitors of RNA polymerase.
b. We have a lead molecule to start with for the development of antimicrobial agents with therapeutic activity.
What is the next step? What work is planned?
As the next step, we plan to perform a structureactivity relationship study through the synthesis of a large number of GPI0363 derivatives; and perform crystal structure analysis of staphylococcal SigA in the presence of GPI0363.
GPI0363 inhibits the interaction of RNA polymerase with DNA in Staphylococcus aureus
Atmika Paudel, Suresh Panthee, Hiroshi Hamamoto and Kazuhisa Sekimizu
RSC Adv., 2019, 9, 37889-37894
DOI: 10.1039/C9RA06844A, Paper
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.










