Hair and nails grow back, but heart valves cannot regenerate naturally. This fact is being challenged by a team of scientists who have developed a method that could someday regrow defective heart valves.
Valvular interstitial cells (VIC), the most prevalent cells in the heart valve, are developmentally locked in a state of quiescence, preventing their division within the body. In people suffering from inflammation-induced or inborn heart valve defects, damaged valves have to be replaced surgically with either mechanical (made with artificial polymers) or bioprosthetic (made with heart tissue) valves.
People with artificial valves are required to take blood thinners for extended periods and make significant changes to their lifestyle. In addition, artificial valves in younger patients do not grow and remodel with time, causing additional complications during adulthood. These issues are further compounded by an estimate suggesting that over 850,000 patients will require heart valve transplants by the year 2050.
Soumen Jana and colleagues at the Division of Cardiovascular Diseases, Mayo Clinic, USA approached this problem from a different angle. Relying on studies showing that VICs can be isolated from heart valves and grown in a laboratory setting, the team developed a nanofibrous membrane-based scaffolding structure to support the growth of VICs. Similar to the cross-cross patterns formed by ropes in a hammock, their polycaprolactone polymer scaffold comprises randomly oriented nanofibers (~457 nm in diameter) to form a cradle within which VICs from defective valves can be grown.
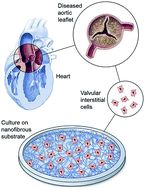
The study found that VICs from healthy valves showed greater levels of cell division on the scaffold compared to VICs from defective valves. Interestingly, the scaffold induced collagen deposition from VICs obtained from both healthy and defective valves. The study also looked at a series of genes and proteins important in VIC growth. Patient derived VICs grown on nanofibrous scaffolds deposited appropriate amounts of cementing proteins necessary for leaflet regeneration.
Clinical trials aimed at regenerating heart valves with chemical drugs and DNA modifying methods are under way. In their study, Jana and colleagues suggest VIC regeneration as a novel idea. They also engineer a scaffold that supports VIC growth, demonstrate its practicality and highlight its ability to be translated into a clinically impactful technology.
Read the full article here:
Regeneration ability of valvular interstitial cells from diseased heart valve leaflets
Soumen Jana, Rebecca Hennessy, Federico Franchi, Melissa Young, Ryan Hennessya and Amir Lerman










