Non-small-cell lung cancer (NSCLC) is among the leading causes of cancer-related deaths globally. Our understanding of the way tumors grow, spread and respond to therapy is driven largely by studies conducted on tumor cells growing as monolayers in plastic cell culture flasks in laboratories across the world. The ability to develop novel and more effective cancer-fighting drugs is dependent, in part, on developing cell culture systems that allow scientists to better observe how tumor cells grow in a three dimensional, physiologically relevant environment.
 |
|
|
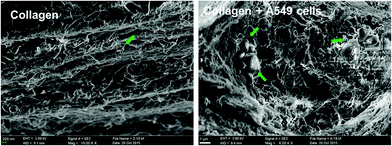
SEM images of the collagen meshwork and A549 cell aggregates (noted by the arrow head) formed during the
3D cultivation in vitro.
|
The tumor microenvironment (TM) is the area that immediately surrounds a tumor and includes non-cancer cells together with secreted proteins called the extracellular matrix (ECM), which supports tumor growth. Monolayer cell cultures, although utilized widely, cannot accurately mimic the TM. For instance, cell-cell and cell-ECM interactions that influence tumor growth cannot be observed in great detail with conventional monolayer cultures. Inspired by the up-and-coming field of tumor engineering, which aims to construct culture models that recapitulate aspects of the TM, a team of researchers led by Dr. Dan-Dan Wang at the Chinese Academy of Sciences developed a 3D culture system wherein A549 cells (immortal lung cancer cells of human origin) grow on a collagen hydrogel scaffold.
To demonstrate the utility of the 3D culture system, the study measured cell viability and showed that cells in the collagen hydrogel scaffold were alive for extended periods (>12 days) in vitro. The study also assessed the appearance of artificial A549 tumors growing on the hydrogel to demonstrate that 3D cultures more closely recapitulate the morphology of tumors growing within human tissues.
The proliferation of A549 cells is driven by the activation of a cell surface protein called Epidermal Growth Factor Receptor (EGFR), which in turn switches on genes that sustain cell growth and cell division. The team observed that Gefitinib, a drug known to disrupt growth-promoting signals arising at EGFR, was able to significantly constrain A549 cell proliferation in 3D cultures. Interestingly, the team reports that a higher concentration of Gefitinib was required to curb cell growth in 3D cultures compared to monolayers due to the complex architecture of the artificial tumors in 3D cultures.
Collectively, this study demonstrates an improved culture model of human lung cancer. Since collagen is an important component of the ECM, the study sets the stage for future efforts to better recapitulate the TM in vitro. The collagen hydrogel scaffold system could serve as in important tool in the discovery of targeted therapies for lung cancer.
Read the full article here: