If asked to name a promising element to contribute to highly efficient, clean energy, few chemists would immediately think of lead.
Meanwhile Yuan-Yuan Feng and colleagues, at Tsinghua University in Beijing, China, have worked with a more conventional catalyst combination in the form of palladium and gold for the same reaction. They found that controlled deposition of Pd on Au nanoparticles could tune the Pd dispersion and produce higher catalytic activity for the electrooxidation of formic acid. They have also characterised in detail the interaction of Pd with the reactive species.
To find out more, read about the work in RSC Advances for free until the 14th March 2013:
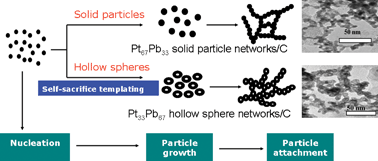
Pt–Pb hollow sphere networks: self-sacrifice-templating method and enhanced activity for formic acid electrooxidation, Xiao Zhao, Jianbing Zhu, Weiwei Cai, Meiling Xiao, Liang Liang, Changpeng Liu and Wei Xing, RSC Adv., 2013, 3, 1763–1767
Catalytic Pd-on-Au nanostructures with improved Pd activity for formic acid electro-oxidation, Yuan-Yuan Feng, Gui-Rong Zhang and Bo-Qing Xu, RSC Adv., 2013, 3, 1748–1752
By Sara Coles










