Scientists from China have identified and characterised the elusive gold(I) hydrates that result from the common gold(I) pre-catalyst Ph3PAuOTf.
Gold(I) complexes have been the subject of a great deal of attention in recent year due to their application in a broad range of chemical reactions and their excellent catalytic activity. Their advantages over some other metal catalysts includes their insensitivity to moisture, therefore not requiring a completely ‘dry’ reaction environment and instead can even be used in reactions with water as the solvent.
Amongst some of the ambiguous aspects of the catalytic species actually involved in these transformations, the exact role of water, and whether it reacts with the gold complexes, is uncertain. This is important in order to gauge the impact water has on the catalytic activity and the turnover frequency. 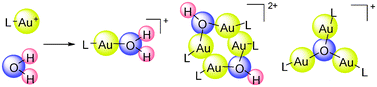
Read the full article for free!
Identification of (phosphine)gold(I) hydrates and their equilibria in wet solutions, Yu Tang and Biao Yu, RSC Adv., 2012, DOI: 10.1039/C2RA22282E
Stay up-to-date with the latest content in RSC Advances by registering for our free table of contents alerts.