A straightforward route to synthesize pyrrolylBODIPY dyes, which can be used for fluorescent imaging, from acid chloride and excess pyrrole has been developed by scientists in China.
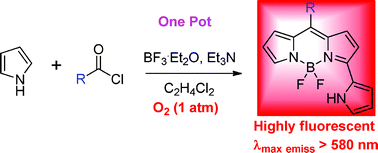
In this work, Erhong Hao, Lijuan Jiao and colleagues from Anhui Normal University, China, report that by reacting acyl chloride and excess pyrrole in dichloromethane under an oxygen atmosphere, to give the pyrrolylBODIPY dyes after about 10 hours. The application of these dyes to the fluorescent imaging of living-cells was then tested and the results suggested that the dyes were non-toxic and could easily be taken up by the cells.
Read the full article for free!
One-pot efficient synthesis of pyrrolylBODIPY dyes from pyrrole and acyl chloride, Min Zhang, Erhong Hao, Yajun Xu, Shengzhou Zhang, Hongnian Zhu, Qi Wang, Changjiang Yu and Lijuan Jiao, RSC Adv., 2012, DOI: 10.1039/C2RA22203E
You may also be interested in these articles…
Synthesis and spectroscopic properties of bodipy dimers with effective solid-state emission, Lizhi Gai, Hua Lu, Bin Zou, Guoqiao Lai, Zhen Shen and Zhifang Li, RSC Adv., 2012, 2, 8840-8846
Thienyl-substituted BODIPYs with strong visible light-absorption and long-lived triplet excited states as organic triplet sensitizers for triplet–triplet annihilation upconversion, Yinghui Chen, Jianzhang Zhao, Lijuan Xie, Huimin Guo and Qiuting Li, RSC Adv., 2012, 2, 3942-3953
Stay up-to-date with the latest content in RSC Advances by registering for our free table of contents alerts.










