A team from Alabama, USA have reported a highly selective extraction of the uranyl ion from aqueous solution via η2 coordination using hydrophobic amidoxime-functionalized ionic liquids.
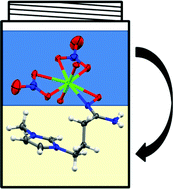
Though well-studied, the coordination of amidoxime to the uranyl ion is not well understood. In this paper, Robin Rogers and his team from the University of Alabama have been able to utilize the functionality of ionic liquids to demonstrate the controversial coordination mechanism for extraction of uranium from seawater by amidoxime extractants. They have demonstrated, through extraction, spectroscopic, and crystallographic studies that hydrophobic, amidoxime-functionalized ionic liquids selectively extract the uranyl ion from aqueous solution via η2 coordination.
Simply register to download the full article here:
Highly selective extraction of the uranyl ion with hydrophobic amidoxime-functionalized ionic liquids via η2 coordination
Patrick S. Barber , Steven P. Kelley and Robin D. Rogers
RSC Adv., 2012, Advance Article
DOI: 10.1039/C2RA21344C