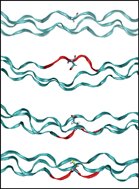
Brittle bone disease – osteogenesis imperfecta (OI) – is a genetic disease that affects more than 1 in 10,000 people. The genetic basis for most forms of the disease lies in the mutation, typically a glycine replacement, in the genes that encode for type I collagen, the major structural component of the extracellular matrix of bone. But the precise molecular mechanisms of how single point mutations can alter the structure of collagen molecules are unknown.
Scientists in the US and Italy have used molecular dynamics simulations to study the effect of OI mutations on the folding of mutant collagen peptides. The work shows that OI mutations lead to local unfolding of the collagen triple helix. Buehler and co-workers provide a possible molecular-level explanations of the current major OI mechanism models available in literature. The results reported is an important step towards a complete understanding of the mechanisms underlying this disease.
Find out more about this research by downloading the RSC Advances article for free. Simply register here for free access.
Osteogenesis imperfecta mutations lead to local tropocollagen unfolding and disruption of H-bond network
Alfonso Gautieri, Simone Vesentini, Alberto Redaelli and Markus J. Buehler
RSC Adv., 2012, Advance Article , DOI: 10.1039/C2RA01047J, Paper
P.S. To keep up to date with our latest articles, sign up to receive our table of content alert or our newsletter.