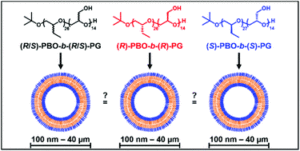
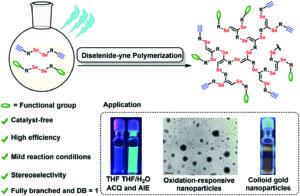
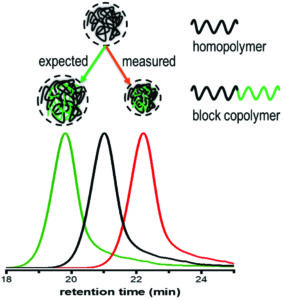
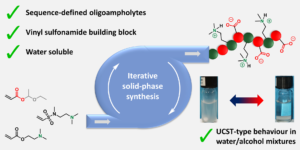
Wehr et al. introduced chirality into aqueous block copolymer (BCP) self-assemblies in a study discriminating the effect of tacticity from that of crystallinity.
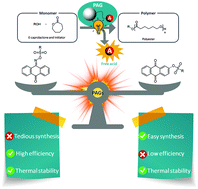
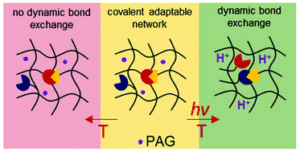
Amphiphilic block copolymers (BCPs) bearing mostly atactic hydrophobic polymers on the main chain have long served as building blocks to produce nanocompartments with diverse morphologies and a variety of (bio)technological applications. More recently, the use of isotactic hydrophobic blocks has attracted significant interest since the effect of stereoregularity of the main chain of BCPs on the formation and morphology of aqueous self-assemblies has not yet been elucidated. Recent studies on isotactic hydrophobic blocks are based on crystallisation-driven self-assembly (CDSA) not allowing to unravel the differences between atactic and isotactic BCPs and associate them with morphological changes in BCP assemblies. CDSA additionally prevents higher complexity and applicability since the formed crystalline membranes generally lack flexibility and fluidity or require handling in temperatures above their melting or glass transition temperatures (Tg) to form well-controlled self-assemblies.
To discriminate the effect of tacticity from that of crystallinity in aqueous self-assemblies of amphiphilic BCPs, Gaitzsch, Meier and collaborators synthesized poly(butylene oxide)-block-poly(glycidol) (PBO-b-PG) BCPs, differing solely in their tacticities (R/S, R and S). Despite the differences in their stereochemistry, all polymers displayed similar thermal and structural behaviour, proving that stereoregularity did not induce crystallinity or formation of secondary structures in bulk or in solution. However, the nanoscopic polymersomes (i.e. small unilamellar vesicles, SUVs) composed of the different BCPs expressed stability differences when studying self-assembly into homogenous phases of SUVs. Interestingly, only the atactic BCPs formed microscopic giant unilamellar vesicles (GUVs) which were stable over several hours while GUVs composed of isotactic BCPs ruptured within minutes after formation. The ability of atactic PBO-b-PG to form microreactors was highlighted by reconstituting the membrane protein OmpF in the GUV membrane via microfluidics and performing an enzyme reaction inside its lumen.
This study differentiates for the first time the effect of tacticity from that of crystallinity in aqueous self-assemblies of amphiphilic BCPs and is expected pave the way in designing versatile vesicles with fluid membranes composed of atactic or isotactic BCPs. Studies of the interplay of membrane chirality with transmembrane proteins or guests in nano- and micro- compartments are now within reach.
Tips/comments directly from the authors:
- Kinetic measurements of the polymerisations of racemic and enantiopure monomers revealed that both enantiomers reacted in the same speed. Hence in case of synthesising the atactic polymer, an ideal statistical distribution of m and r diads is achieved.
- The microfluidic technique applied in here was essential to form the GUVs. Other approaches to form GUVs were not successful due to the instability of the self-assemblies especially of the stereoregular polymers.
- We essentially took commercially available but expensive enantiopure monomers to generate highly stereoregular polymers, which formed less stable (i.e. inferior) self-assemblies than the cheaply accessible atactic polymers. However, it allowed us to falsify the common believe that a higher order in polymers always leads to better defined structures or a higher stability.
Citation to the paper: Fully amorphous atactic and isotactic block copolymers and their self-assembly into nano- and microscopic vesicles, Polym. Chem., 2021,12, 5377-5389.
Link to the paper: https://pubs.rsc.org/en/content/articlelanding/2021/py/d1py00952d
 Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.