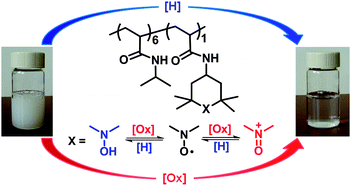
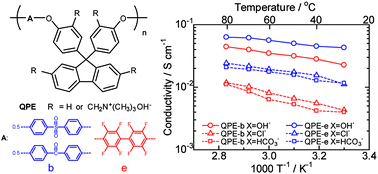
We welcome Professor Brent Sumerlin to the Polymer Chemistry Editorial Board.

Professor Brent S. Sumerlin graduated with a B.S. from North Carolina State University (1998) and obtained a Ph.D. in Polymer Science and Engineering at the University of Southern Mississippi (2003) under the direction of Prof. Charles L. McCormick. After serving as a Visiting Assistant Professor at Carnegie Mellon University under the direction of Professor Krzysztof Matyjaszewski (2003-2005), he joined the Department of Chemistry at Southern Methodist University (Dallas, Texas, USA) as an assistant professor in 2005 and was promoted to associate professor in 2009. Professor Sumerlin has received several awards, including an Oak Ridge Associated Universities Ralph E. Powe Award (2007), an NSF CAREER Award (2009), an ACS Leadership Development Award (2010), and an Alfred P. Sloan Research Fellowship (2010). Current research in his group involves the synthesis of functional macromolecules, responsive polymer systems, polymer-protein bioconjugates, and dynamic covalent macromolecular assemblies.
Read Professor Sumerlin’s article in Polymer Chemistry:
Conjugation of RAFT-generated polymers to proteins by two consecutive thiol–ene reactions
Ming Li, Priyadarsi De, Hongmei Li and Brent S. Sumerlin
Polym. Chem., 2010, 1, 854-859
Comments Off on Meet our new Editorial Board member