Selenium-containing block copolymers and their oxidation-responsive aggregates by Ning Ma, Ying Li, Huifeng Ren, Huaping Xu, Zhibo Li and Xi Zhang is the paper featured on the front cover of Polymer Chemistry issue 10.
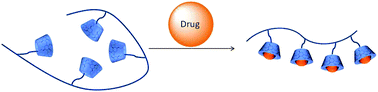
The paper reports self-assembled responsive aggregates made from selenium-containing polymers. These polymer aggregates are more sensitive to oxidants than sulfur-containing analogues. The team hope this behavior could form the basis of a new drug delivery system.
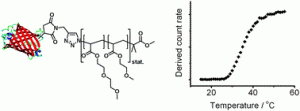
The polymer is a selenium-containing amphiphilic block copolymer with a hydrophobic polyselenide block flanked by two hydrophilic poly(ethylene glycol) blocks.
Interested to know more? Why not read the full article for free: Selenium-containing block copolymers and their oxidation-responsive aggregates.














![NNCAJWITQ3CA65OY3CCAD7BD1XCAI6NT9ICAW3ZTGSCA6UKW2OCABNZQMDCAULS386CA6FMIXMCAB11870CARE9YIDCA7KR95BCA2FI983CAR2LVGNCAIAGBWUCAOVSEOBCAL2S7RFCAZX9DLKCA272FMF Graphical abstract: Probing cucurbit[8]uril-mediated supramolecular block copolymer assembly in water using diffusion NMR](https://blogs.rsc.org/py/files/2010/10/NNCAJWITQ3CA65OY3CCAD7BD1XCAI6NT9ICAW3ZTGSCA6UKW2OCABNZQMDCAULS386CA6FMIXMCAB11870CARE9YIDCA7KR95BCA2FI983CAR2LVGNCAIAGBWUCAOVSEOBCAL2S7RFCAZX9DLKCA272FMF.gif)